Abstract
Endogenous ciliary neurotrophic factor (CNTF)1 regulates neurogenesis of the adult brain in the hippocampal subgranular zone (SGZ)2 and the subventricular zone (SVZ)3. We have previously shown that the cAMP-inhibiting D2 dopamine receptor increases neurogenesis by inducing astroglial CNTF expression. Here, we investigated the potential role of CNTF in the proliferative response to pharmacological stimulation of the serotonin 1A (5-HT1A)4 receptor, which also inhibits cAMP, in adult mice and rats. Like others, we show that systemic treatment with the active R-enantiomer of the 5-HT1A agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT)5 induces proliferation in the SGZ in rats using unbiased stereology of 5-Bromo-2′-deoxyuridine (BrdU)6 positive nuclei. However, despite the bioactivity of R-8-OH-DPAT, as also shown by a decrease in hippocampal nNOS7 mRNA levels, it did not increase CNTF mRNA as shown by highly specific quantitative RT-PCR (qPCR)8. Surprisingly, R-8-OH-DPAT did not cause an increase in SVZ proliferation in rats or in either the SVZ or SGZ of two different strains of mice, C57BL/6J, and 129SvEv, using acute or chronic treatments. There also were no changes in CNTF mRNA, and also not in mice treated with a widely used racemic mixture of 8-OH-DPAT, higher doses or after intracerebral injection, which reduced nNOS. In contrast to the others, we propose that the 5-HT1A receptor might be non-functional in mice with regards to regulating normal neurogenesis and has region-selective activities in rats. These species- and region-specific actions raise important questions about the role of the 5-HT1A receptor in human neurogenesis and its implications for the field of depression.
Keywords: 5-HT1a receptor, 8-OH-DPAT, CNTF, nNOS, proliferation, 5-HT1A agonist
1. Introduction
Neurogenesis in the adult mammalian brain occurs predominantly in the SVZ of the anterior lateral ventricles and the SGZ of the hippocampal dentate gyrus (Hagg, 2009; Kriegstein and Alvarez-Buylla, 2009; Ming and Song, 2005; Soumier et al., 2010; Suh et al., 2009). SVZ neuroblasts migrate along the rostral migratory stream to the olfactory bulb and SGZ neuroblasts migrate into the neighboring granule cell layer where they become integrated neurons or die (Lois and Alvarez-Buylla, 1994; Luskin, 1993; Winner et al., 2002).
Many endogenous molecules regulate adult CNS neurogenesis (Bath and Lee, 2010; Hagg, 2005, 2009; Lie et al., 2004; Ming and Song, 2005; Suh et al., 2009). CNTF is responsible for ~30% of SVZ neurogenesis as shown in CNTF−/− mice and by antibody injections (Emsley and Hagg, 2003; Yang et al., 2008). We study CNTF because it is produced almost exclusively in the nervous system (Ip, 1998; Stockli et al., 1989) and would therefore be a good target for indirect stimulation of neurogenesis by systemic small molecule drugs. We found that nigrostriatal projections induce neurogenesis via D2 dopamine receptors, which is entirely mediated by astroglial CNTF (Baker et al., 2004; Yang et al., 2008). This is consistent with the fact that D2 receptors inhibit cAMP in astrocytes (Kalkman et al., 2003; Vallar and Meldolesi, 1989), whereas CNTF expression is inhibited by cAMP in astrocytes (Carroll et al., 1993; Rudge et al., 1994). CNTF is predominately produced by astrocytes (Dallner et al., 2002; Sendtner et al., 1994) which are spared in most neurodegenerative diseases. Therefore, they are ideal pharmacological targets to serve as CNTF "factories" to enhance endogenous proliferation. This would also circumvent the peripheral side effects and low bioavailability seen with systemic administration of CNTF (Thoenen and Sendtner, 2002).
We wanted to find additional cAMP-inhibiting drugs to increase CNTF, as they could be combined with low doses of D2 agonists to reduce systemic drug doses and, consequently, side effects. The 5-HT1A receptor is expressed on a subset of astroglia throughout the brain, in addition to neurons in the raphe nuclei (Whitaker-Azmitia et al., 1993). Their activation decreases cAMP levels (Azmitia, 2001; Kalkman et al., 2003; Mendez et al., 1999; Vanhoose et al., 2004). Colocalization of 5-HT1A receptor and GFAP is among the highest in the polymorphic layer of the dentate gyrus (Whitaker-Azmitia et al., 1993). Interestingly, serotonergic and dopaminergic projections to the SVZ overlap where neurogenesis and high expression of CNTF are located (Hagg, 2005). Activation of 5-HT1A receptor increases neurogenesis in the SVZ and SGZ in rats (Banasr et al., 2004; Brezun and Daszuta, 1999; Huang and Herbert, 2005; Soumier et al., 2010). A landmark study (Santarelli et al., 2003) showed that hippocampal neurogenesis in 129SvEv mice and the effects of antidepressants are regulated through 5-HT1A receptors, using 5-HT1A receptor −/− mice and 8-OH-DPAT. Acute but not chronic 8-OH-DPAT treatment increases SGZ proliferation in C57BL/6J mice (Klempin et al., 2010). Here, we tested whether CNTF mediates the proliferative effects of 8-OH-DPAT in the SVZ and SGZ of adult C57BL/6J and 129SvEv mice, as compared to rats.
2. Material and methods
2.1 Animals
All animal procedures were performed according to University of Louisville Institutional Animal Care and Use Committee protocols and the National Institutes of Health guidelines. In addition, every effort was made to minimize animal suffering and reduce the number of animals used.
A total of 153 mice were used, i.e., 101 C57BL/6J adult male mice (8–12 weeks, 20–30g, stock # 000664, Jackson Laboratory, Bar Harbor, ME, USA), 40 129SvEv adult male mice (model # 129SVE-M [129S6/SvEvTac], 11 weeks, 20–30 g, Taconic, Hudson, NY, USA), and 12 wild type background of our CNTF mice (essentially C57BL/6) were used. In addition, 10 adult male Sprague–Dawley rats (280–350g, Harlan, Indianapolis, IN) were used. The average weight of the animals in each experimental group was the same. All invasive procedures in mice were performed under deep anesthesia obtained by an intraperitoneal injection of 0.4 mg/g body weight Avertin (2,2,2-tribromoethanol in 0.02 ml of 1.25% 2-methyl-2-butanol in saline, Sigma-Aldrich, St Louis, MO, USA). Rats were anesthetized with an intramuscular injection of a mixture (62.5 µg/g weight of the animal) containing 5 ml of ketamine (500 mg/ml, Hospira, Lake Forest, IL), 0.5 ml of acepromazine (10 mg/ml, ButlerSchein, Dublin, OH), and 1.2 ml xylazine (20 mg/ml, Akorn, Decantur, IL) diluted in 13.3 ml of 0.9% saline.
2.2 Systemic 5-HT1A agonist treatments and BrdU injection protocols
The animals were given saline, (R)-(+)-8-OH-DPAT dissolved in saline (1 mg/kg/day, Cat # H-140; Sigma-Aldrich, St. Louis, MO), a full and specific 5-HT1A agonist and the active enantiomer of (±)-8-OH-DPAT, or racemic mixture 8-OH-DPAT (0.1, 1 or 10 mg/kg/day, Cat.# H8520; Sigma-Aldrich, St. Louis, MO). Drugs were administered either i.p. once daily for 3 days or s.c. via Alzet pump (Cat # 1003D with a flow rate of 1 µl/day for 3 days, or Cat # 1002 with a flow rate of 0.25 µl/day for 14 days or Cat #: 1004 with a flow rate of 0.11 µl/day for 28 days; Durect Corp., Cupertino, CA). The 14 day pumps were replace on day 14 with a new 14-day pump with fresh reagent for the 28-day infusion in C57BL/6J mice. BrdU (Cat #: B5002, Sigma-Aldrich, St. Louis, MO) was injected at 50 mg/kg twice daily for 3 days with the final injection 2 hours before processing for histology.
2.3 Direct microinjections of R-8-OH-DPAT next to the SVZ and SGZ
A custom micropipette of approximately 50 µm diameter at the tip (P-97 Flaming/Brown Micropipette Puller, Sutter Instrument Company, Novato, CA) was affixed to the needle of a 10 µl Hamilton syringe and used to inject 1 µl of a 2.75 µg/µl solution of R-8-OH-DPAT or saline into each injection site. This dose was calculated from the dosage used for a 24 hour infusion which decreased nNOS mRNA (Zhang et al., 2010). The injection site located at stereotaxic coordinates from Bregma: 0.5 rostral, 2.3 lateral, and 1.5 below the dura for the area next to the SVZ and 2.3 caudal, 2.5 lateral, and 2.5 below the dura for the area next to the HF in the mouse (Franklin and Paxinos, 1996). Four hours after injection, the SVZ and HF were collected as described in Section 2.4.
2.4 mRNA measurements by qPCR
To obtain total RNA, each sample was isolated from freshly dissected 0.5 mm wide SVZ strips or the entire HF9, flash frozen using liquid nitrogen, and isolated from a commercial kit (Cat #: 74104, Qiagen, Valencia, CA). Briefly, 1 µg RNA was treated with DNAse (Cat #: 18068-015, Invitrogen, Carlsbad, CA) and used as templates for reverse transcription, which included 1 µl of 0.5 µg/µl random primers (Cat#: C1181,Promega, Madison, WI), 1 µg total RNA, 5 µl of 5× buffer, 1.25 µl of 10 mM dNTP mix, 2.25 µl RNAse free water, and 1 µl (200 units) of Moloney Murine Leukemia Virus Reverse Transcription (M-MLV RT, Cat #: M170, Promega, Madison, WI) and heated at 37°C for 1 hr. Water was used as a no-template control instead of total RNA. The qPCR was performed using primer sets specific for CNTF (Mm00446373_m1 and Rn00755092_m1), Nos1 (Mm00435175_m1 and Rn00583793_m1), HTR1A (5-HT1A receptor; Mm00434106_s1 and Rn00561409_s1), GapDH (4352339E), and Ywhaz (Rn00755072) (all from Applied Biosystems, Carlsbad, CA).
2.5 Histology
Mice were transcardially perfused with ice-cold PBS, pH 7.4, until the liver became clear (approx. 10–20 ml) followed by 10 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were dissected out and immediately placed in ice-cold 4% paraformaldehyde to postfix overnight followed by cryoprotection in 30% sucrose in 0.1 M phosphate buffer overnight. Serial coronal brain sections of 30 µm thick were cut on a Leica SM 2000R freezing stage sliding microtome and stored in anatomical order in 24-well plates filled with Millonig’s buffer. Double immunofluorescent staining for glial fibrillary acidic protein, clone GA5 (GFAP) (MAB3402, 1:1000, Millipore, Billerica, MA) and 5-HT1A receptor (AB5406, 1:300, Millipore, Billerica, MA) was performed using appropriate secondary antibodies conjugated with Alexa488 (1:500 goat anti-mouse) and Alexa546 (1:500; goat anti-guinea pig; Molecular Probes; Eugene, OR). For BrdU staining, starting at a random point along the rostrocaudal axis of the brain sections stained included those approximately between stereotaxic coordinates (caudal to Bregma) 1.18 to 0.14 mm for SVZ and −1.34 to −3.08 mm for HF in the mouse (Franklin and Paxinos, 1996). Sections for rats were approximately between 1.6 to 0.2 mm for SVZ and −2.3 to −4.52 mm for HF in the rat (Paxinos and Watson, 1986). Every sixth section (180 µm apart) through the SVZ and HF was immunostained against BrdU (MAB3510, mouse IgG, clone BU-1, 1:100,000; Millipore, Billerica, MA). Briefly, brain sections were incubated in 50% formamide in 2X SSC at 65°C for 2 h, rinsed in fresh 2X SSC, inc ubated in 2N HCl at 37°C for 30 min, neutralized in 0.1 M boric acid, pH 8.5, for 10 min. Next, sections were sequentially incubated in 10% normal horse serum for 30 min, primary antibody overnight at 4°C, biotinylated horse anti-mouse IgG (1:800; Vector Laboratories, Burlingame, CA) for 1 hr, and avidin-biotin complex conjugated with peroxidase for 1 hr (1:600; Vector Laboratories). All incubations were carried out at room temperature unless otherwise noted. In between steps, the sections were rinsed 3 × 10min. The chromogen reaction was performed with 0.04% 3, 3’-diaminobenzidine (D5637, Sigma-Aldrich) solution containing 0.06% nickel ammonium sulfate and 1% hydrogen peroxide in 0.05 M Tris buffer-HCl. Sections were then rinsed in 0.1 M phosphate buffer, mounted on gelatin coated glass slides in anatomical order, and cover slipped in Permount (SP15, Thermo Fisher Scientific, Pittsburg, PA).
2.6 Unbiased stereological counts and statistics
The number of BrdU+ nuclei in the SVZ of each brain was estimated using a motorized Leica DMIRE2 microscope and an unbiased optical fractionator stereological method (Stereologer; Stereology Resource Center, Chester, MD) (Baker et al., 2004). For the SVZ, the reference space was defined as an ~50 µm-wide strip of the entire lateral of all the lateral ventricle encompassing dorsoventrally the ventral tip and the dorsolateral triangular regions of the lateral ventricle, rostrocaudally from the genu of the corpus callosum to the caudal end of the decussation of the anterior commissure, and laterally the boundary between the SVZ and striatum. Within the reference space, BrdU-positive nuclei were counted in defined frames (frame size: 2000 µm2, frame height: 15 µm, guard height: 2 µm, frame spacing: 100 µm) and the total number of BrdU-positive cells in a brain was calculated by the software as: n = number of nuclei counted × 1/section sampling fraction × 1/area sampling fraction × 1/thickness sampling fraction. A modified unbiased stereology protocol was used to count the BrdU+ cells in the SGZ of the dentate gyrus (West et al., 1991). Briefly, the total number of BrdU+ cells between the granule cell layer and hilus of the dentate gyrus that were within two cells body distance from the granular layer of the dentate gyrus was counted bilateral from the stereological coordinates previous stated. The total number of cells is calculated by the modified formula for rare events: n = number of nuclei counted × 1/section sampling fraction = total number of cells counted × (1/ (1/6)). All analysis was done bilaterally except for in rats, in which a unilateral count was performed due to the other half of the brain was used for mRNA analysis. All analyses were performed blindly. Statistical analyses were performed with the Student's unpaired t-test using Excel software (Microsoft, Redmond, WA) or ANOVA with Tukey post-hoc, where appropriate, using SPSS software (IBM Corporation, Armonk, NY). A value of p ≤ 0.05 was considered statistically significant.
3. Results
3.1 5-HT1A receptor co-localization in SVZ astrocytes in C57BL/6J mice
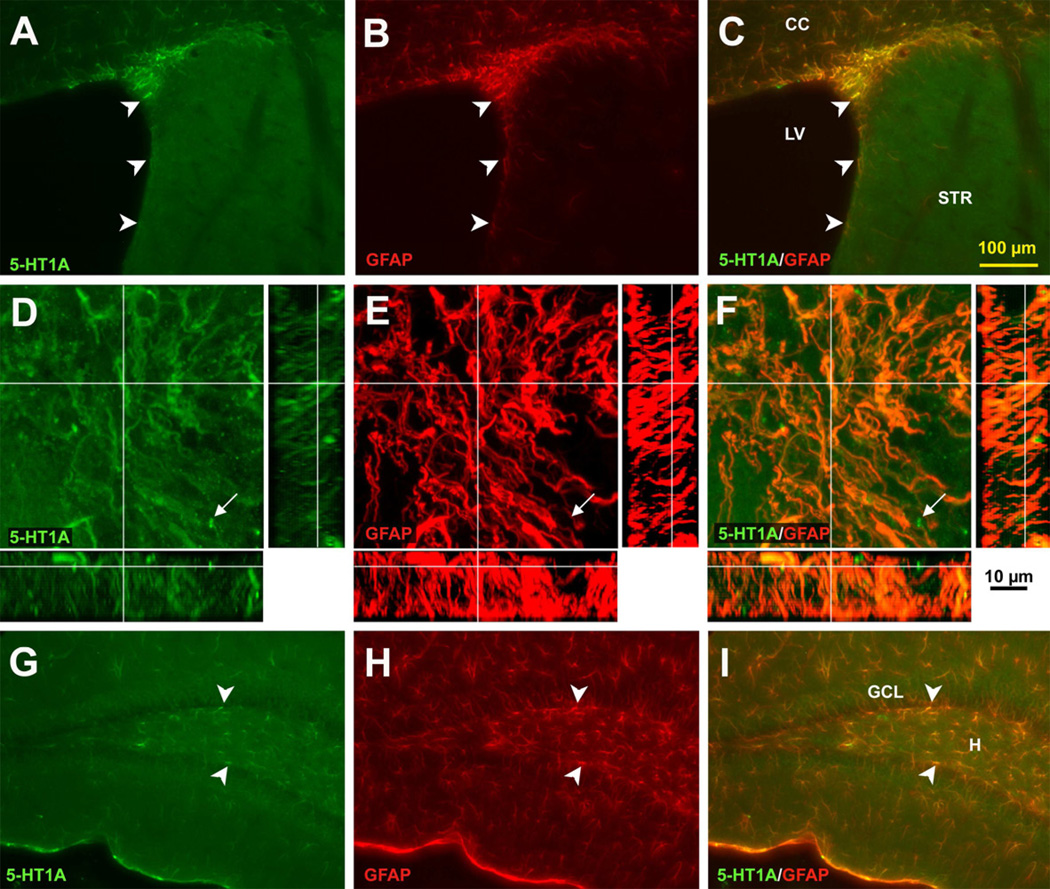
Astrocytes in different areas of the brain are heterogeneous populations that can express different amounts and types of receptors (Emsley and Macklis, 2006). Interestingly, the 5-HT1A receptor is found in highest abundance in astrocytes of rats in the two regions of the brain where neurogenesis occurs throughout life (Whitaker-Azmitia et al., 1993). In mice, 5-HT1A receptor staining also co-localized with GFAP in the SVZ (Figure 1A–C). This was confirmed in confocal microscopy z-stack images showing that most of 5-HT1A receptor staining is co-localized with GFAP in addition to a few putative axon terminals, presumably presynaptic receptors of serotonergic projections (arrows in Figure 1D–F). However, colocalization with synaptophysin remains to be done. In the dentate gyrus, 5-HT1A receptor immunostaining is also co-labeled with GFAP, including the SGZ (Figure 1G–I). We have previously shown that only very few GFAP+ cells also have BrdU labeling (Emsley and Hagg, 2003). Here, all the GFAP+ cells were co-localized with 5-HT1A receptor immunostaining; therefore, these GFAP+/5-HT1A receptor+ cells consist of primarily the regular astrocytes as well as potentially the neural stem cells.
Figure 1. 5-HT1A receptors are present in astrocytes in the SVZ and SGZ of adult C57BL/6J mice.
5-HT1A receptor (A) and GFAP (B) are co-localized (C) in the SVZ. Confocal microscopy shows that 5-HT1A receptor (D) and GFAP (E) are almost exclusively co-localized (F), as indicated in the z-stack, except for a few putative axon terminals, presumably presynaptic receptors of serotonergic projections (arrows). In the dentate gyrus, 5-HT1A receptor (G) and GFAP (H) also shows co-localization (I), including in the SGZ. Scale bar 100 µm (A–C, H–I), 10 µM (D–F). CC = corpus callosum, GCL = granule cell layer, H = hilus, LV = lateral ventricle, STR = striatum.
3.2 5-HT1A agonist R-8-OH-DPAT does not increase proliferation in adult C57BL/6J mice
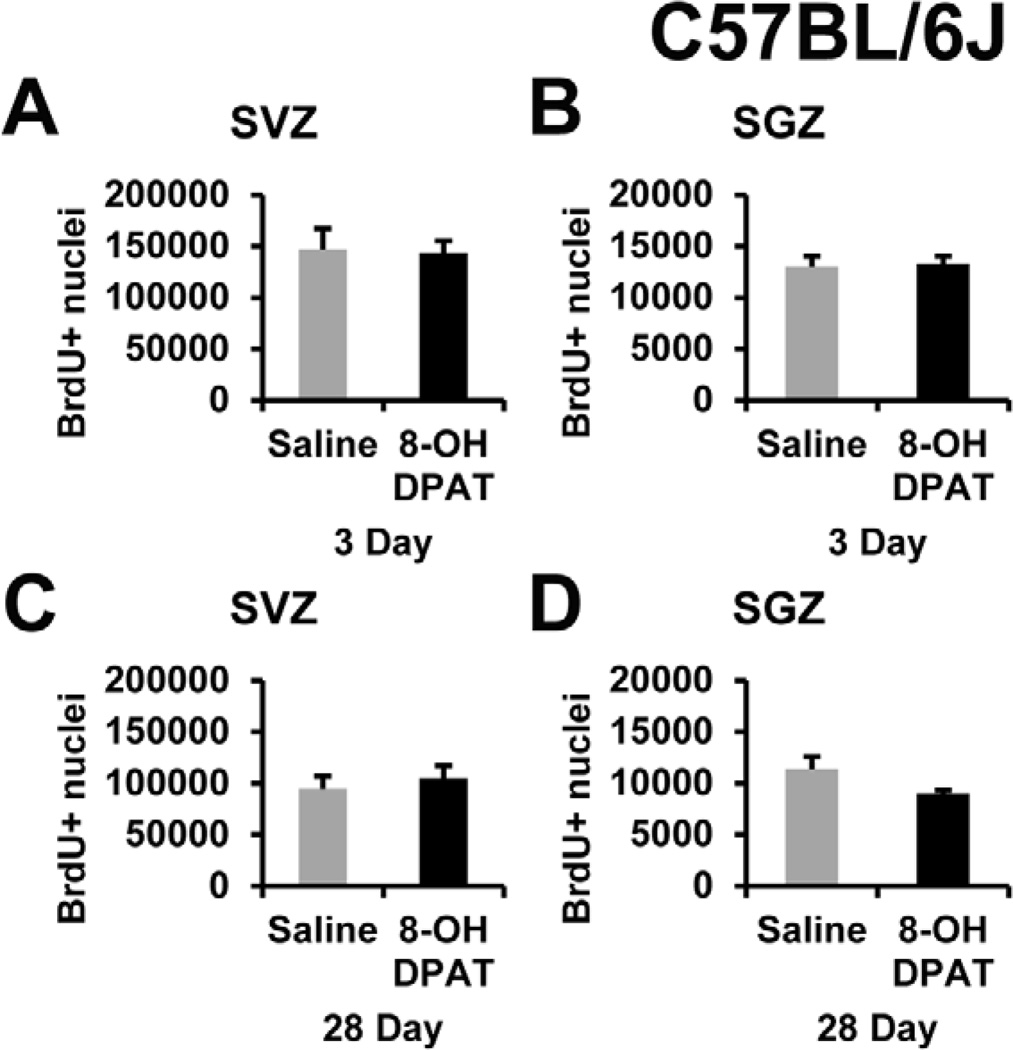
We intended to test the role of 5-HT1A receptor directly in CNTF−/− mice and their littermates but wanted to first test R-8-OH-DPAT in less expensive C57BL/6J mice. The latter are the most commonly used strain in neuropharmacological research and our normal strain that we currently and previously have used to show changes in SVZ proliferation after 3 day drug treatments (Emsley and Hagg, 2003; Yang et al., 2008). Male C57BL/6J adult mice received 1 mg/kg/daily over 3 days using subcutaneous Alzet osmotic mini-pump containing saline or saline plus the active enantiomer of 8-OH-DPAT. This dose was used by others who have shown 8-OH-DPAT to promote neurogenesis in both rats and mice (Santarelli et al., 2003; Soumier et al., 2010). BrdU was injected twice daily at 50 mg/kg i.p. per injection. After an unbiased stereological analysis, we found no differences in either BrdU+ cells in the SVZ (Figure 2A) or SGZ (Figure 2B). Separately, we used higher doses and found that 3 or 10 mg/kg per day bolus injections in the wild type background of our CNTF mice (essentially C57BL/6) also did not induce proliferation (data not shown; n=4 per group). The osmotic pump was chosen in order to give a constant level of drug over the 3-day drug treatment in light of the 26 min half-life of 8-OH-DPAT in the brain (Perry and Fuller, 1989). Others have shown that the SGZ neurogenesis was increased following 28-day osmotic pump administration of 1 mg/kg/day by osmotic pump (Santarelli et al., 2003). Therefore, C57BL/6J mice received saline or R-8-OH-DPAT for 28-days using two 14-day Alzet osmotic pumps. To identify proliferating cells, BrdU was injected twice-daily 50mg/kg i.p. over the last 3 days with the last injection two hours before sacrifice. The R-8-OH-DPAT mice did not show an increase in BrdU+ nuclei in the SVZ (Figure 2C) or SGZ (Figure 2D). Lastly, we did not observe the typical serotonergic behavior (forepaw treading, flat body posture, and weaving of the head) that has been described in rats (Tricklebank et al., 1985) in any of these R-8-OH-DPAT-treated mice.
Figure 2. 5-HT1A receptor agonist R-8-OH-DPAT does not increase proliferation in C57BL/6J mice.
R-8-OH-DPAT was administered s.c. with a dose of 1 mg/kg delivered via Alzet osmotic pump for either 3 days or by two 14-day Alzet pumps for a total of 28 days to C57BL/6J male mice. There were no significant changes in BrdU+ nuclei acutely after 3 days, in the SVZ (A) or SGZ (B) or chronically after 28 days in the SVZ (C) or SGZ (D). BrdU was given i.p. twice daily for 3 consecutive days with the last injection 2 hours before euthanasia and processing for histology. n= 5, 6 in each group for 3 day and 28 day, respectively.
3.3 R-8-OH-DPAT does not increase CNTF expression in C57BL/6J mice
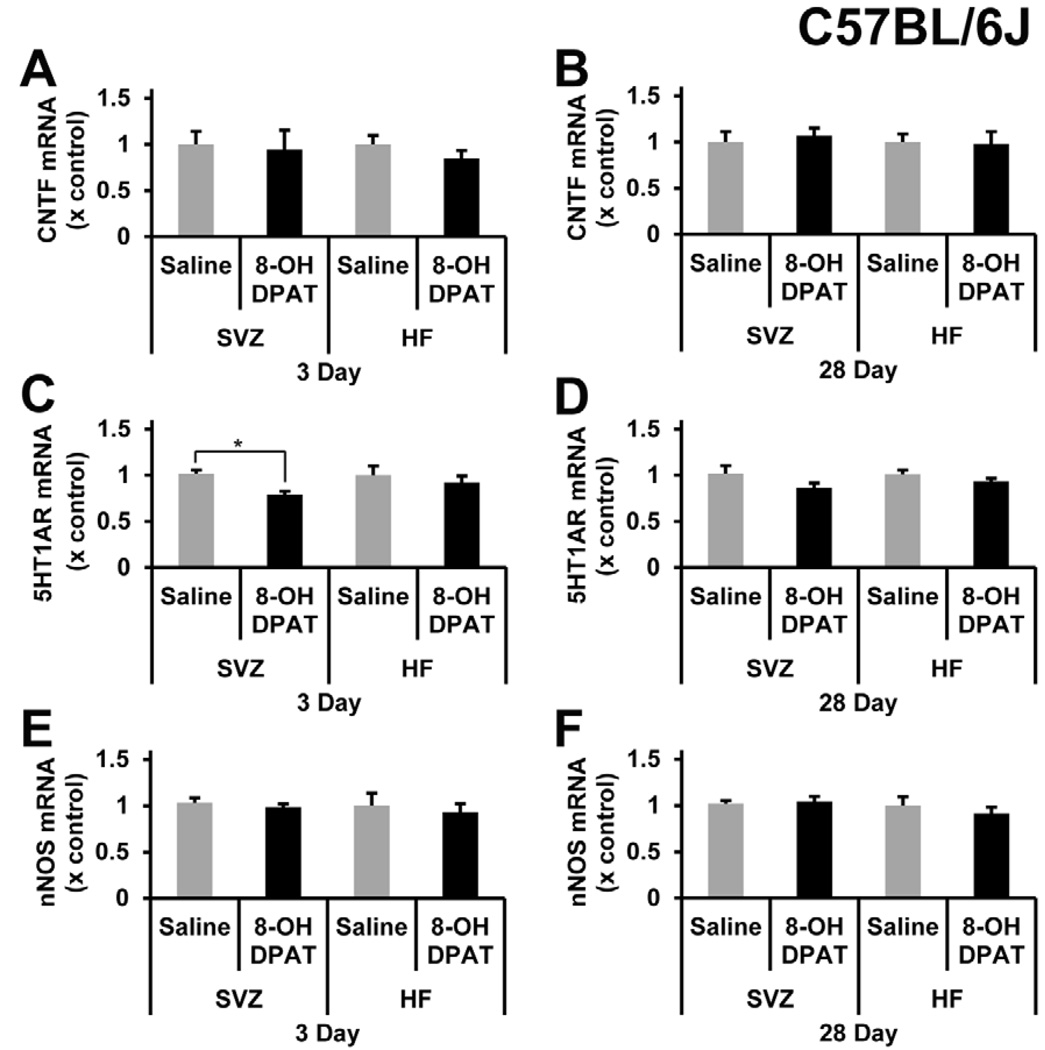
It was possible that R-8-OH-DPAT failed to induce proliferation because it does not induce CNTF. We had shown that increases in CNTF cause increased neurogenesis (Emsley and Hagg, 2003; Yang et al., 2008). There were no changes in CNTF mRNA in the SVZ and HF either acutely after 3-day infusion (Figure 3A) or chronically after 28-day infusion (Figure 3B) in C57BL/6J mice. In the same tissue, we measured 5-HT1A mRNA to confirm there was not a down-regulation of the receptor after the administration of R-8-OH-DPAT. After 3 days infusion, 5-HT1A was decreased by ~20% in the SVZ (Figure 3C), but not the HF (Figure 3D). Lastly, to find additional evidence for the expected effects of R-8-OH-DPAT, we measured nNOS mRNA levels in the same tissue, as this was shown by others to decrease in the hippocampus of B6129SF2 mice 1 day after treatment and to diminish completely by 7 days (Zhang et al., 2010). However, in our C57BL/6J mice, nNOS mRNA did not decrease after acute (Figure 3E) or chronic (Figure 3F) systemic administration of R-8-OH-DPAT.
Figure 3. R-8-OH-DPAT does not alter CNTF or nNOS mRNA, but can reduce 5-HT1A receptor mRNA levels in C57BL/6J mice.
CNTF mRNA levels were not changed acutely (A), after 3 days, or chronically (B), after 28 days of 5-HT1A receptor agonist treatment in the SVZ and HF. R-8-OH-DPAT did cause a significant decrease in 5-HT1A receptor mRNA levels (C) (p=0.0499) in the SVZ but not the HF after 3 days of 1 mg/kg of R-8-OH-DPAT. 5-HT1A receptor mRNA levels did not change in the SVZ or HF after 28 days of R-8-OH-DPAT infusion (D). nNOS mRNA levels in the SVZ and HF did not significantly change acutely (E) or chronically (F). * signifies p<0.05, n= 4, 5, 6 in each group for saline 3 day, R-8-OH-DPAT 3 day, and 28 day, respectively.
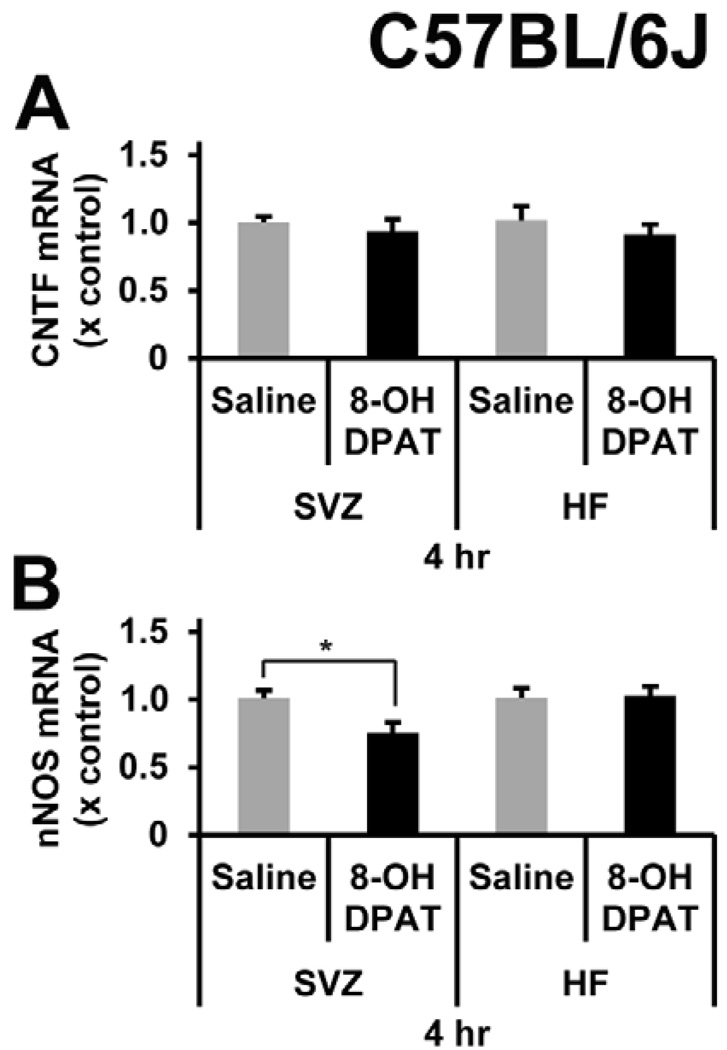
We wanted to further investigate the bioactivity of R-8-OH-DPAT in the mouse brain by intracerebral injections as was done to show decreases in nNOS (Zhang et al., 2010). In addition, a direct injection away from the midbrain serotonergic nuclei and a short time frame to the collection of the tissue reduces the likelihood of influence from the 5-HT1A autoreceptors. Here, we injected R-8-OH-DPAT in the area next to the SVZ and SGZ which resulted in a decrease (25%) in nNOS mRNA after only 4 hours in the SVZ but not in the HF (Figure 4A). However, there were no changes in CNTF mRNA levels at 4 hours (Figure 4B). nNOS mRNA was unchanged from controls in the SVZ or HF at 24 hours (data not shown, n = 5).
Figure 4. R-8-OH-DPAT decreases nNOS mRNA levels, but not CNTF mRNA levels.
A direct microinjection of R-8-OH-DPAT into the area adjacent to the SVZ and SGZ at a dose of 2.75 µg per site per animal. The tissue was collected at 4 hours post injection. A) There were no differences in CNTF mRNA levels in the SVZ or in the HF. B) There was a significant decrease of 25% in the SVZ in nNOS mRNA. There were no changes in the nNOS mRNA levels in the HF. * signifies p<0.05, n= 5 in each group.
3.4 R-8-OH-DPAT does not stimulate proliferation or CNTF in adult 129SvEv mice
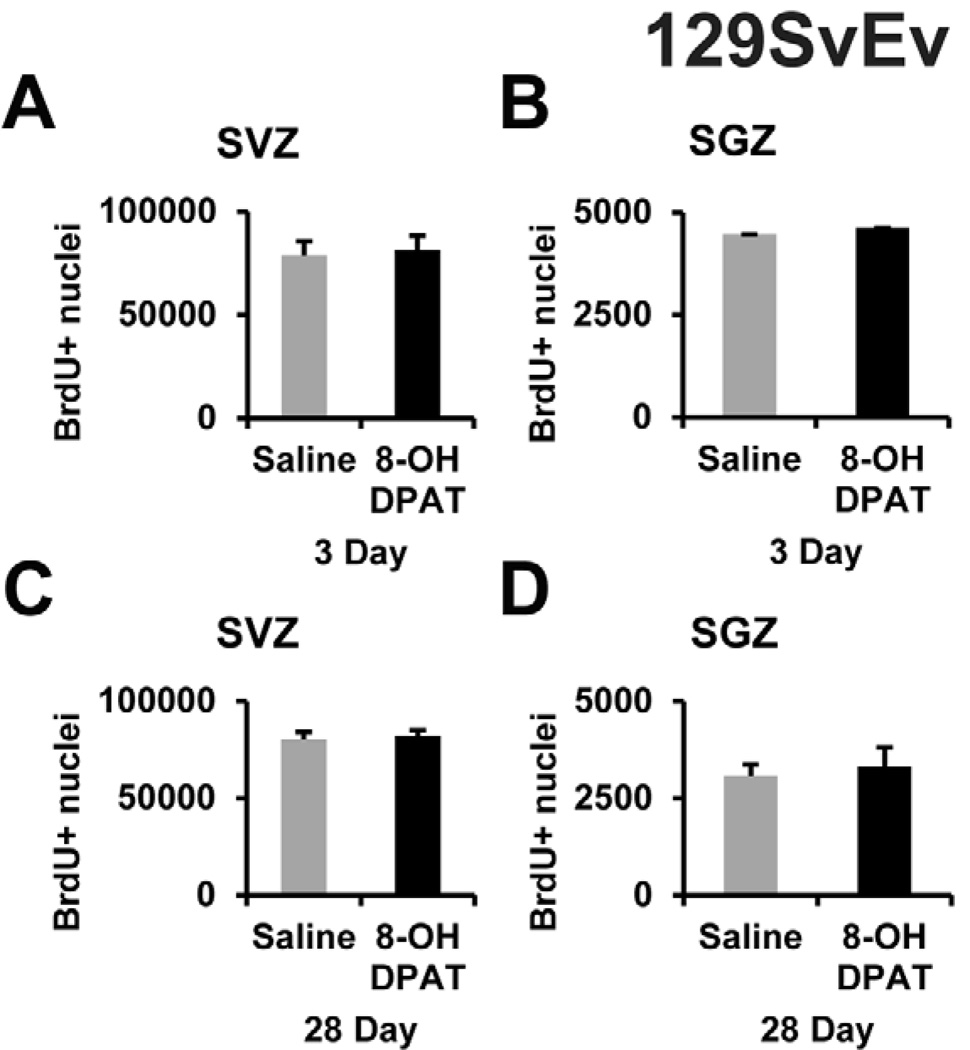
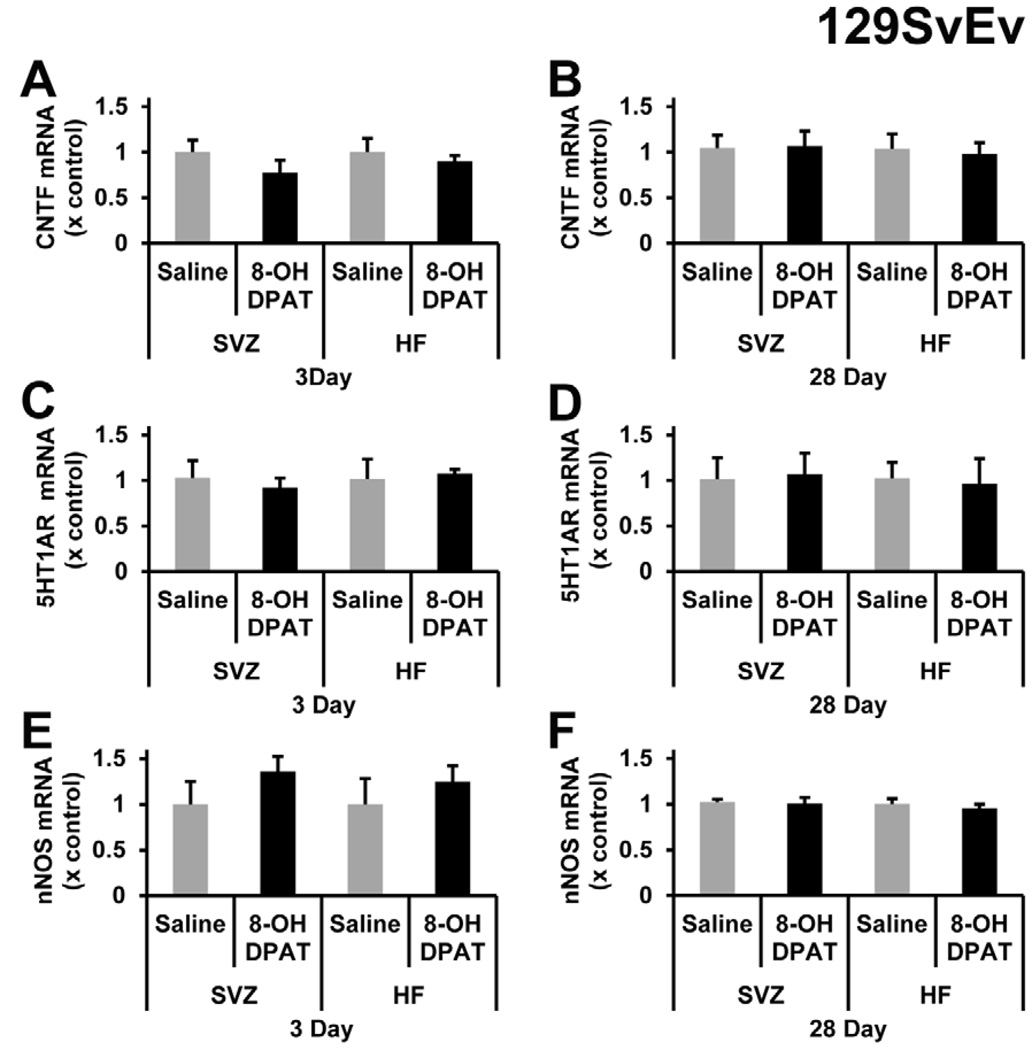
It is possible that our negative results after R-8-OH-DPAT treatment were related to the C57BL/6J strain. We, therefore, repeated the R-8-OH-DPAT experiment of the study by Santarelli et al. (2003), in which they used male 129SvEv mice to show that (±)-8-OH-DPAT increased neurogenesis specifically through 5-HT1A receptor. Here, male 129SvEv mice from the same vender were first treated with a 3-day i.p. injection of saline of 1 mg/kg R-8-OH-DPAT. There was no significant change in the BrdU+ nuclei for the SVZ (Figure 5A) or SGZ (Figure 5B). Given the fact that the Santarelli et al. (2003) infused for 28 days using Alzet pumps, we also infused saline or the same dose, 1 mg/kg/day, of R-8-OH-DPAT. There was no significant change seen in BrdU+ nuclei in either the SVZ (Figure 5C) or SGZ (Figure 5D). Since we did not see changes in proliferation, we measured CNTF mRNA levels and found no effects in either the SVZ or the HF of acute (Figure 6A) or chronic (Figure 6B) R-8-OH-DPAT treatment groups. 5-HT1A receptor mRNA levels were not affected after acute (Figure 6C) or the chronic (Figure 6D) treatments in the SVZ or SGZ. As in the C57BL/6J mice, the nNOS mRNA levels for both acute (Figure 6E) and chronic (Figure 6F) groups were not significantly affected by the R-8-OH-DPAT treatment. As in the C57BL/6J mice, we did not observe the typical serotonergic behavior in any of the 8-OH-DPAT-treated 129SvEv mice.
Figure 5. R-8-OHDPAT does not increase proliferation in the SVZ or SGZ in 129SvEv mice.
R-8-OH-DPAT was administered with a dose of 1 mg/kg delivered either by daily i.p. injection for 3 days or s.c. via Alzet osmotic pump for 28 day to 129SvEv male mice. There were no changes in the number of BrdU+ nuclei in the SVZ (A) or SGZ (B) acutely or the SVZ (C) or SGZ (D) chronically. n= 5 in each group.
Figure 6. R-8-OH-DPAT does not alter mRNA levels of CNTF, 5-HT1A receptor or nNOS in 129SvEv mice.
CNTF mRNA levels were not changed acutely (A), after 3 days, or chronically (B), after 28 days of treatment in the SVZ and HF. R-8-OH-DPAT did not cause a significant decrease in 5HT1A mRNA levels in the SVZ or HF after 3 days (C) or 28 days (D) of 1 mg/kg of R-8-OH-DPAT. nNOS mRNA levels in the SVZ and hippocampal formation did not significantly change acutely (E) or chronically (F). n= 5 in each group.
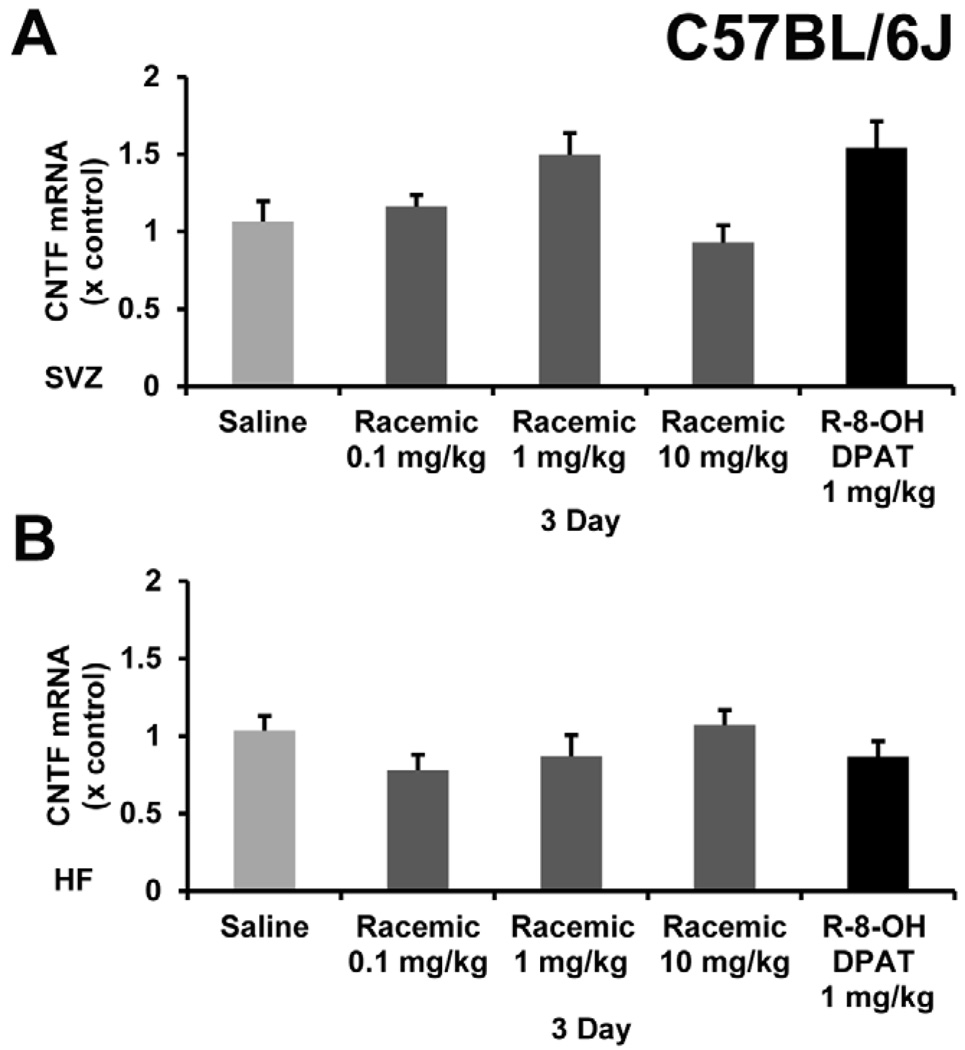
Our initial goal was to find another drug to combine with a D2 agonist that would increase CNTF resulting in increased neurogenesis in the CNS as shown with the D2 agonist alone (Baker et al., 2004; Yang et al., 2008). Since the racemic mixture of 8-OH-DPAT was found to increase neurogenesis in the HF (Santarelli et al., 2003), we wanted to also test it along side of the active enantiomer in C57BL/6 mice. Bolus i.p. injections of either 0.1, 1 or 10 mg/kg of the racemic 8-OH-DPAT or 1 mg/kg of R-8-OH-DPAT once daily for 3 days. The tissues were collected 2 hours after the final injection. Consistent with the rest of our data with the specific R-enantiomer, the racemic mixture failed to cause significant increases in CNTF mRNA in the SVZ or HF over a range of doses (Figure 7). As in the R-enantiomer, we did not observe the typical serotonergic behavior in any of these doses.
Figure 7. Racemic 8-OH-DPAT does not change CNTF mRNA levels in the SVZ or HF in C57BL/6J mice.
CNTF mRNA levels did not significantly change in either the SVZ (A) or HF (B) after administering the racemic mixture of 8-OH-DPAT at doses of 0.1, 1, and 10 mg/kg/day by daily i.p. injection for 3 days in C57BL/6J mice. R-8-OH-DPAT was given at 1 mg/kg/day as a control for comparison to the previous experiments.
3.5 R-8-OH-DPAT increases proliferation in adult rats, but only in the SGZ and not through CNTF
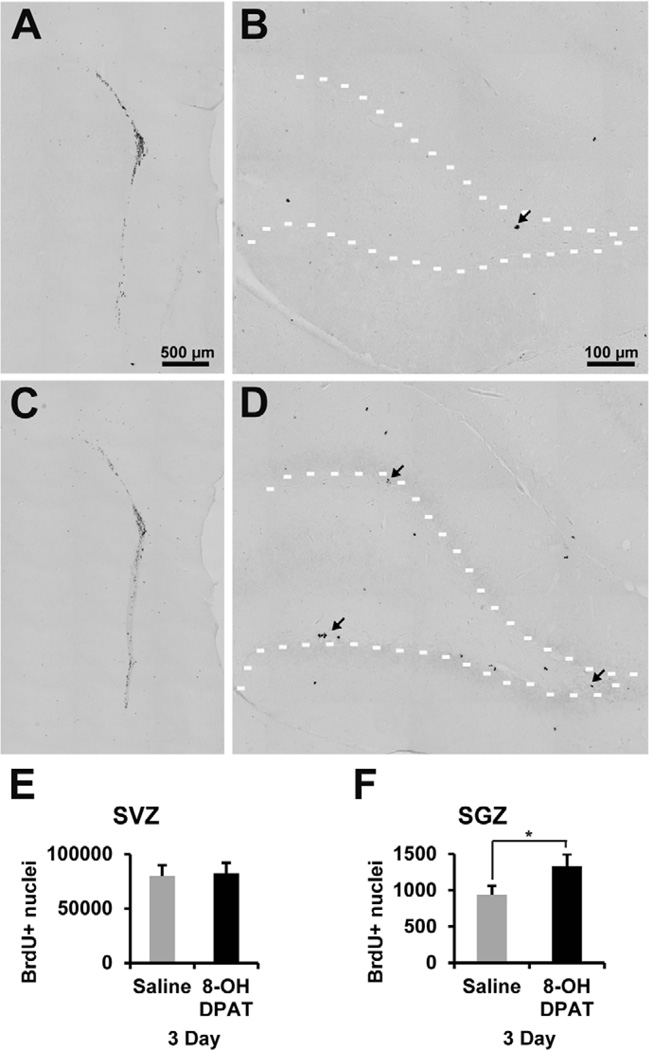
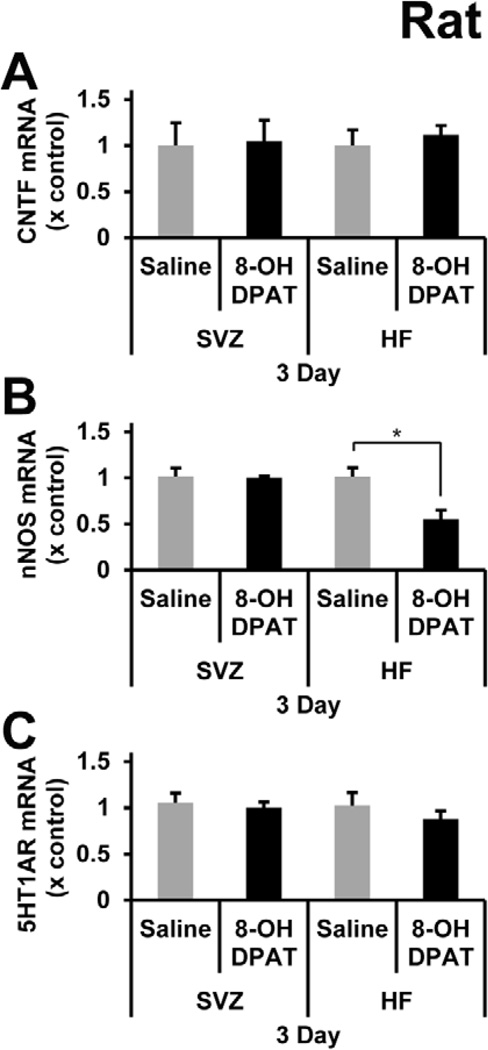
Despite some evidence for bioactivity of R-8-OH-DPAT in C57BL/6J mice as shown by the decrease in 5-HT1A receptor mRNA after acute systemic treatment or reduced nNOS mRNA after intracerebral injection close to the SVZ, we wanted to further verify that R-8-OH-DPAT could elicit biological responses. The literature on the use of 8-OH-DPAT in rats is much more extensive, including behavioral effects (Tricklebank et al., 1985) and its ability to increase hippocampal neurogenesis (Banasr et al., 2004; Huang and Herbert, 2005; Soumier et al., 2010). Thus, male Sprague-Dawley rats were injected daily i.p. with saline or 1 mg/kg R-8-OH-DPAT over 3 days. Within minutes of R-8-OH-DPAT injection the rats exhibited typical serotonergic behavior, verifying that our drug was effective at eliciting a serotonergic response. The number of BrdU+ nuclei in the SGZ was increased by 43% after the 3-day treatment (Figure 8B, D, and F). In contrast, the SVZ showed no change in the number of BrdU+ nuclei (Figure 8A, C, and E). Since we found that R-8-OH-DPAT increased proliferation in the rat SGZ, we assessed whether this was mediated by an increase in CNTF. However, there were no changes in CNTF mRNA levels in the SVZ or HF (Figure 9A). To test whether the difference between the SGZ and SVZ proliferation was related to the presence of functional 5-HT1A receptor, we measured the effects of R-8-OH-DPAT on nNOS mRNA levels. The nNOS mRNA levels were decreased by 50% in the HF, but were not affected in the SVZ (Figure 9B). Lastly, 5-HT1A receptor mRNA levels were checked to see if there was a down regulation of the receptor. 5-HT1A receptor mRNA was not significantly changed in the SVZ or SGZ (Figure 9C).
Figure 8. R-8-OH-DPAT causes an increase in proliferation in the SGZ of Sprague-Dawley rats.
R-8-OH-DPAT was administered with a dose of 1 mg/kg delivered by daily i.p. injection for 3 days in Sprague-Dawley rats. BrdU labeling in the SVZ (A) and SGZ (B) of saline treated rats. BrdU labeling in the SVZ (C) and SGZ (D) of R-8-OH-DPAT treated rats. There was no significant difference in the number of BrdU+ nuclei in the SVZ of rats (E). There was a significant 43% increase in the number of BrdU+ nuclei in the SGZ in rats (F). * signifies p<0.05, n= 5 in each group. Scale bar 500 µm (A and C) and 100 µm (B and D).
Figure 9. R-8-OH-DPAT induces rat SGZ proliferation, decreases nNOS mRNA levels, but does not increase CNTF mRNA levels.
A) There was no difference in CNTF mRNA levels in the SVZ or in the HF, where proliferation was observed in the SGZ after R-8-OH-DPAT treatment. B) There was a significant decrease in nNOS mRNA levels only in the HF and not in the SVZ. C) There were no changes in 5-HT1A receptor mRNA levels in the SVZ or HF. * signifies p<0.01, n= 5 in each group.
4. Discussion and Conclusion
Like others, we find that R-8-OH-DPAT increases proliferation in the adult rat SGZ (Banasr et al., 2004; Huang and Herbert, 2005; Soumier et al., 2010). We wanted to define the role of CNTF in this neurogenic response, given the commonality between 5-HT1A and D2 receptors, i.e., both lowering cAMP and found on astrocytes. CNTF seems not involved in the proliferative response to R-8-OH-DPAT, as it did not increase in the rat HF. The lack of changes in CNTF expression in rats and mice, and in the SVZ and HF suggests that, unlike the D2 receptor (Yang et al., 2008), 5-HT1A receptor may not inhibit cAMP in astrocytes in vivo. A recent study showed that the R-isoform of 8-OH-DPAT did not decrease forskolin-stimulated increases in cAMP in the membranes isolated from rat dorsal raphe nucleus (Valdizan et al., 2010). Others have shown that 5HT1A receptors in cultured astrocytes do not couple functionally to adenylyl cyclase (Hirst et al., 1998). However, further study is needed to conclude that R-8-OH-DPAT does not decrease cAMP in the SVZ or SGZ.
Besides its classical inhibition of adenylyl cyclase (Cornfield et al., 1991), 5-HT1A receptor also can couple to other signaling pathways such as phospholipase C (Fargin et al., 1989), mitogen-activated protein kinases (Della Rocca et al., 1999) and AKT (Cowen et al., 2005), which play a role in adult neurogenesis (Kinsler et al., 2010; Ma et al., 2009; Manning et al., 2010). These pathways could signal independent of adenylyl cyclase or converge on different adenylyl cyclase subtypes to increase or decrease cAMP (Raymond et al., 1999). Although the exact intracellular mechanism of action of R-8-OH-DPAT on proliferation in the rat SGZ remains to be determined, the finding that it does not act through CNTF should enable the development of drug combinations. For example, one could expect an additive effect on the levels of SGZ neurogenesis in the rat by combining 5-HT1A receptor agonist and D2 agonists, the latter stimulating neurogenesis by increasing CNTF (Yang et al., 2008).
If not CNTF, what then might regulate the proliferative response to 5-HT1A receptor agonist in the rat SGZ? The difference between the proliferative response in the SVZ and SGZ and its correlation with the decrease in nNOS mRNA levels suggests that 5-HT1A receptor activation in the SGZ decreases nNOS signaling, which results in an increase in proliferation. In fact, NO appears to reduce proliferation and nNOS inhibition in the CNS of adult mammals causes an increase in proliferation in the SVZ (Matarredona et al., 2005) and HF (Gass and Riva, 2007; Gur et al., 2007; Nakagawa et al., 2002a; Nakagawa et al., 2002b). In cultured hippocampal neurons, 8-OH-DPAT was shown to decrease nNOS, which decreases NO production, increasing phosphorylation of CREB10 which presumably increases transcription (Zhang et al., 2010). Furthermore, in CREBαΔ mutant mice there was a 52% increase in BrdU+ nuclei in the SGZ compared to wild-type mice (Gur et al., 2007). Moreover, a decrease in neurogenesis was observed when CREB activity was down regulated by expressing a dominant-negative CREB (Nakagawa et al., 2002a; Nakagawa et al., 2002b). Pharmacological inhibition of NO by L-NAME11 was shown to increase neurogenesis in the SVZ of adult mice (Romero-Grimaldi et al., 2008). This suggests that since there were no changes observed in the mRNA levels of nNOS after systemic R-8-OH-DPAT treatment in the SVZ of rats and the SVZ and SGZ of mice that 5-HT1A receptor does not modulate their nNOS levels. Alternatively, the changes might be too transient as we saw a decrease in nNOS 4 hrs but not 24 hours after microinjection in the mouse SVZ.
In rats, R-8-OH-DPAT was shown to increase BrdU+ nuclei in the SVZ 4 hours after i.p. injection although the data for longer times were not reported (Banasr et al., 2004). Our 3-day treatments did not result in any changes in SVZ BrdU+ nuclei. Other than strain differences, we do not know the reason for this apparent discrepancy. Thus, the reason for the lack of a proliferative effect of R-8-OH-DPAT in the rat SVZ remains to be determined. The presence of 5-HT1A receptors in the SVZ suggests that they may not be functional in the SVZ astrocytes under normal conditions. Others have shown that astrocytes from 2-day old Sprague-Dawley rat pups cultured from various other brain regions express non-functional 5-HT1A receptor in culture (Hirst et al., 1998). If so, this would suggest that astrocytes in different CNS regions, e.g. SVZ vs. SGZ, can function entirely different, as also suggested by their different phenotypes (Emsley and Macklis, 2006). Another possibility is that the 5-HT1A receptor in the two different neurogenic regions of the brain have differences in intracellular coupling and/or signaling as it has been shown with different cell types in vitro (Raymond et al., 1999). The activity of 5-HT1A receptor was shown to be impacted by alterations in any of the guanine nucleotide-binding protein G(i), alpha subunit (Giα), in particular, alterations in the levels of Giα1 was shown to have a greater impact on 5-HT1A receptor activity (Lin et al., 2002; Liu et al., 1999; Valdizan et al., 2010). It remains to be determined whether there are different levels of the Gi/Go protein isoforms accessible to the 5-HT1A receptor for coupling in the SVZ versus the SGZ, but it would explain why there is an apparent difference in functional activity. Another possibility is that R-8-OH-DPAT acts on other, differently expressed, receptors in the two structures. The R-enantiomer of 8-OH-DPAT is considered to be the prototypical selective agonist for 5-HT1A receptor as shown by radio ligand binding (Middlemiss and Fozard, 1983). However, the use of transgenic mice and pharmaceutical inhibitors have shown that 8-OH-DPAT has agonistic activity on 5-HT7 (Hedlund et al., 2004) and α2-adrenoceptors (Heusler et al., 2010). Finally, the difference in the neurogenic response between the SVZ and SGZ most likely represents the different physiological roles of the two systems, and differences in the serotonergic neurons that innervate these regions. In turn, it means that hippocampal proliferation can be stimulated by 5-HT1A receptor agonists without affecting the olfactory proliferation, perhaps opening up opportunities for region-selective drug treatments to increase new neuron formation.
Our results also point to remarkable differences between rats and mice with regards to the 5-HT1A receptor’s function and its role in proliferation. Our data suggest that R-8-OH-DPAT can have bioactivity in mice (e.g., reduced SVZ nNOS mRNA 4hr after intracerebral injection) but by and large is without an effect. We considered the possibility that 8-OH-DPAT caused a negative feedback on the 5-HT1A receptor via 5-HT1A autoreceptors in rat (Blier et al., 1998) and thereby did not increases in proliferation or in CNTF. The intracerebral injections close to the SVZ and SGZ were also aimed at circumventing potential stimulation of the 5HT1A autoreceptors in the midbrain nuclei which contain the serotonergic cell bodies. The lack of consistent effects, including the lack of behavioral effects over a range of doses, which are so obvious in rats, suggests that the receptors are not fully functional in these strains of mice. Previously, 8-OH-DPAT administration was shown to induce hypothermia in mice and rats to a different extent, suggesting the 5-HT1A receptor heterogeneity is present between mice and rats (Moser, 1991).
In their landmark paper,Santarelli et al. (2003) report that the same dose and duration of racemic 8-OH-DPAT causes an increase in BrdU+ nuclei in the SGZ of 129SvEv mice but not in 5-HT1A receptor −/− mice. The proliferative effect of racemic 8-OH-DPAT was replicated in 129SvEv mice within the same laboratory (B. Samuels, personal communication). A few differences might explain the apparent discrepancy between our studies. First, we used the active enantiomer whereas they used the racemic mixture of 8-OH-DPAT. It is unlikely that the S-enantiomer, a partial agonist, would have stimulated proliferation through other receptors or mechanisms, as it was ineffective in 5-HT1A receptor −/− mice (Santarelli et al., 2003). Moreover, we tested different systemic doses of the racemic mixture over 3 days and, like the R-enantiomer, it also did not change CNTF mRNA levels. The most likely difference is the stress caused by their behavioral testing resulted in a depressed baseline of neurogenesis in controls. Stress can have both deleterious and stimulatory properties depending on the type (Sahay and Hen, 2007; Schoenfeld and Gould, 2011). The 129SvEv mice might be unique as the neurogenic effect of fluoxetine (Santarelli et al., 2003) is not seen in BALB/cJ mice within the same laboratory (Holick et al., 2008) or BALB/cJ and C57BL/6J mice in another laboratory (Navailles et al., 2008). Our results are consistent with the finding that a 7 day treatment of 8-OH-DPAT did not increase proliferation whereas a single injection increased proliferation by 55% at 24 hr in the SGZ of C57BL/6 mice (Klempin et al., 2010). Our 3-day data suggest that more than a single injection might be ineffective as a proliferative treatment. Whatever the differences, we propose that 5-HT1A receptor does not play a role in normal proliferation in adult mice, although it has a clear role in rats. In fact, 5-HT1A receptor −/− mice show no differences in the number of BrdU+ nuclei under baseline conditions (Santarelli et al., 2003). Our goal was to target endogenous CNTF in the neurogenic niches using a D2 agonist in combination with another CNTF stimulating drug in order to increase normal proliferation and neurogenesis. Therefore, we find that it would not be productive to further pursue the study of proliferation or CNTF in regards to 8-OH-DPAT and the 5-HT1A receptor in mice.
In light of the differences between rats and mice, what are the potential implications for the role of 5-HT1A receptor in proliferation in humans? This has important implications for the depression field because of the unresolved debate about whether or not changes in neurogenesis play a role in depression (Kempermann, 2008; Thomas and Peterson, 2008). 5-HT1A receptor protein has an 89% homology between rats and humans, 94% between rats and mice, and 86% between mice and humans (Clustal W Software, European Bioinformatics Institute, Cambridge, UK). However, there could be differences in the ligand binding regions or availability of coupling intracellular proteins. Also, rats and humans have a different neuroanatomical distribution in 3H-8-OH-DPAT binding (Duncan et al., 1998). Rats have high binding in the dentate gyrus molecular layer whereas humans have relatively low binding. Humans and rats have high binding in CA-1 stratum radiatum, but only humans have high binding in the CA-1 pyramidal cell layer. These differences could play a role in augmenting or diminishing the proliferative effects seen in rats and make translation to humans more uncertain. We propose that based on the major species differences much more research is needed to define the potential role of 5-HT1A receptor in human hippocampal neurogenesis and the consequences of such a speculative mechanism for clinical depression.
Highlights.
-
>
5HT1A agonist 8-OH-DPAT does not affect proliferation in mice
-
>
8-OH-DPAT increases hippocampal proliferation in rats
-
>
This increase is not mediated by CNTF but possibly by decreased nNOS
-
>
8-OH-DPAT does not change proliferation in the subventricular zone
-
>
Species and regional differences question relevance of 5HT1A to human neurogenesis
Acknowledgements
We wish to thank Chiharu Lovins, Rollie Reid (1972–2011), Christine Yarberry, Kim Fentress, Erin Welsh, Hillary Conway and Kim Jenkins-Milton for their excellent technical assistance. Dr. Peng Yang is thanked for his help with the 5-HT1A images. We very much appreciate the helpful comments and detailed information about the 8-OH-DPAT experiments in the laboratory of Dr. Rene Hen as provided by Dr. Ben Samuels. This work was supported by NIH grants AG29493 and RR15576, Norton Healthcare, and the Commonwealth of Kentucky Challenge for Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CNTF: ciliary neurotrophic factor
SGZ: subgranular zone
SVZ: subventricular zone
5-HT1A: serotonin 1A
8-OH-DPAT: 8-Hydroxy-2-(di-n-propylamino)tetralin
BrdU: 5-Bromo-2′-deoxyuridine
nNOS: neuronal Nitric Oxide Synthase
qPCR: quantitative real-time reverse transcription-polymerase chain reaction
HF: hippocampal formation
CREB: cAMP response element binding protein
L-NAME: N5-[imino(nitroamino)methyl]-L-ornithine, methyl ester, monohydrochloride
References
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Research Bulletin. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol. 2010;70:339–349. doi: 10.1002/dneu.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Annals of the New York Academy of Sciences. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Carroll P, Sendtner M, Meyer M, Thoenen H. Rat ciliary neurotrophic factor (CNTF): gene structure and regulation of mRNA levels in glial cell cultures. Glia. 1993;9:176–187. doi: 10.1002/glia.440090303. [DOI] [PubMed] [Google Scholar]
- Cornfield LJ, Lambert G, Arvidsson LE, Mellin C, Vallgarda J, Hacksell U, Nelson DL. Intrinsic activity of enantiomers of 8-hydroxy-2-(di-n-propylamino)tetralin and its analogs at 5-hydroxytryptamine1A receptors that are negatively coupled to adenylate cyclase. Mol Pharmacol. 1991;39:780–787. [PubMed] [Google Scholar]
- Cowen DS, Johnson-Farley NN, Travkina T. 5-HT receptors couple to activation of Akt, but not extracellular-regulated kinase (ERK), in cultured hippocampal neurons. J Neurochem. 2005;93:910–917. doi: 10.1111/j.1471-4159.2005.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner C, Woods AG, Deller T, Kirsch M, Hofmann HD. CNTF and CNTF receptor alpha are constitutively expressed by astrocytes in the mouse brain. Glia. 2002;37:374–378. [PubMed] [Google Scholar]
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem. 1999;274:4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR, Crews FT, Little KY. Species differences in regional patterns of 3H-8-OH-DPAT and 3H-zolpidem binding in the rat and human brain. Pharmacol Biochem Behav. 1998;60:439–448. doi: 10.1016/s0091-3057(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2:175–186. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargin A, Raymond JR, Regan JW, Cotecchia S, Lefkowitz RJ, Caron MG. Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem. 1989;264:14848–14852. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1996. [Google Scholar]
- Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays. 2007;29:957–961. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP Response Element-Binding Protein Deficiency Allows for Increased Neurogenesis and a Rapid Onset of Antidepressant Response. J. Neurosci. 2007;27:7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15:20–27. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8- OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Heusler P, Rauly-Lestienne I, Tourette A, Tardif S, Ailhaud MC, Croville G, Cussac D. Actions of the prototypical 5-HT1A receptor agonist 8-OH-DPAT at human alpha2- adrenoceptors: (+)8-OH-DPAT, but not (-)8-OH-DPAT is an alpha2B subtype preferential agonist. Eur J Pharmacol. 2010;640:8–14. doi: 10.1016/j.ejphar.2010.04.034. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Cheung NY, Rattray M, Price GW, Wilkin GP. Cultured astrocytes express messenger RNA for multiple serotonin receptor subtypes, without functional coupling of 5-HT1 receptor subtypes to adenylyl cyclase. Brain Res Mol Brain Res. 1998;61:90–99. doi: 10.1016/s0169-328x(98)00206-x. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience. 2005;135:803–813. doi: 10.1016/j.neuroscience.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Ip NY. The neurotrophins and neuropoietic cytokines: two families of growth factors acting on neural and hematopoietic cells. Ann N Y Acad Sci. 1998;840:97–106. doi: 10.1111/j.1749-6632.1998.tb09553.x. [DOI] [PubMed] [Google Scholar]
- Kalkman HO, Feuerbach D, Lötscher E, Schoeffter P. Functional characterization of the novel antipsychotic iloperidone at human D2, D3, [alpha]2C, 5-HT6, and 5-HT1A receptors. Life Sciences. 2003;73:1151–1159. doi: 10.1016/s0024-3205(03)00419-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kinsler R, Taylor MM, Flores NM, Leffert JJ, Beech RD. Altered response to antidepressant treatment in FoxG1 heterozygous knockout mice. Synapse. 2010;64:169–171. doi: 10.1002/syn.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Babu H, De Pietri Tonel D, Alarcon E, Fabel K, Kempermann G. Oppositional effects of serotonin receptors 5-HT1a, 2 and 2c in the regulation of adult hippocampal neurogenesis. Frontiers in Molecular Neuroscience. 2010;3:12. doi: 10.3389/fnmol.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annual Review of Neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annual Review of Pharmacology and Toxicology. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lin SL, Setya S, Johnson-Farley NN, Cowen DS. Differential coupling of 5-HT(1) receptors to G proteins of the G(i) family. Br J Pharmacol. 2002;136:1072–1078. doi: 10.1038/sj.bjp.0704809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Ghahremani MH, Rasenick MM, Jakobs KH, Albert PR. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Galphai protein expression. Gi subtype specificity of the 5-HT1A receptor. J Biol Chem. 1999;274:16444–16450. doi: 10.1074/jbc.274.23.16444. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-Distance Neuronal Migration in the Adult Mammalian Brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Ma DK, Ponnusamy K, Song MR, Ming GL, Song H. Molecular genetic analysis of FGFR1 signalling reveals distinct roles of MAPK and PLCgamma1 activation for selfrenewal of adult neural stem cells. Mol Brain. 2009;2:16. doi: 10.1186/1756-6606-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EE, Ransome MI, Burrows EL, Hannan AJ. Increased adult hippocampal neurogenesis and abnormal migration of adult-born granule neurons is associated with hippocampal-specific cognitive deficits in phospholipase C-beta1 knockout mice. Hippocampus. 2010 doi: 10.1002/hipo.20900. [DOI] [PubMed] [Google Scholar]
- Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Role of nitric oxide in subventricular zone neurogenesis. Brain Research. 2005;49:355–366. doi: 10.1016/j.brainresrev.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Mendez J, Kadia TM, Somayazula RK, El-Badawi KI, Cowen DS. Differential Coupling of Serotonin 5-HT1A and 5-HT1B Receptors to Activation of ERK2 and Inhibition of Adenylyl Cyclase in Transfected CHO Cells. Journal of Neurochemistry. 1999;73:162–168. doi: 10.1046/j.1471-4159.1999.0730162.x. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. European Journal of Pharmacology. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Moser PC. The effect of putative 5-HT1A receptor antagonists on 8-OH-DPAT-induced hypothermia in rats and mice. Eur J Pharmacol. 1991;193:165–172. doi: 10.1016/0014-2999(91)90032-l. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002a;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002b;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. The Journal of Comparative Neurology. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. Academic Press; San Diego: 1986. [etc.] [DOI] [PubMed] [Google Scholar]
- Perry KW, Fuller RW. Determination of brain concentrations of 8-hydroxy-2-(di-npropylamino) tetralin by liquid chromatography with electrochemical detection. Biochem Pharmacol. 1989;38:3169–3173. doi: 10.1016/0006-2952(89)90609-6. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. The recombinant 5- HT1A receptor: G protein coupling and signalling pathways. Br J Pharmacol. 1999;127:1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Grimaldi C, Moreno-Lopez B, Estrada C. Age-dependent effect of nitric oxide on subventricular zone and olfactory bulb neural precursor proliferation. The Journal of Comparative Neurology. 2008;506:339–346. doi: 10.1002/cne.21556. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Morrissey D, Lindsay RM, Pasnikowski EM. Regulation of ciliary neurotrophic factor in cultured rat hippocampal astrocytes. Eur J Neurosci. 1994;6:218–229. doi: 10.1111/j.1460-9568.1994.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature Neuroscience. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M, Carroll P, Holtmann B, Hughes RA, Thoenen H. Ciliary neurotrophic factor. J Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- Soumier A, Banasr M, Goff LK, Daszuta A. Region- and phase-dependent effects of 5-HT(1A) and 5-HT(2C) receptor activation on adult neurogenesis. European Neuropsychopharmacology. 2010;20:336–345. doi: 10.1016/j.euroneuro.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in Adult Neurogenesis. Annual Review of Cell and Developmental Biology. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Peterson DA. Even neural stem cells get the blues: evidence for a molecular link between modulation of adult neurogenesis and depression. Gene Expr. 2008;14:183–193. [PMC free article] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Middlemiss DN, Fozard JR. Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985;117:15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- Valdizan EM, Castro E, Pazos A. Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int J Neuropsychopharmacol. 2010;13:835–843. doi: 10.1017/S1461145709990940. [DOI] [PubMed] [Google Scholar]
- Vallar L, Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989;10:74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- Vanhoose AM, Ritchie MD, Winder DG. Regulation of cAMP levels in area CA1 of hippocampus by Gi/o-coupled receptors is stimulus dependent in mice. Neuroscience Letters. 2004;370:80–83. doi: 10.1016/j.neulet.2004.07.093. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Clarke C, Azmitia EC. Localization of 5-HT1A receptors to astroglial cells in adult rats: implications for neuronal-glial interactions and psychoactive drug mechanism of action. Synapse. 1993;14:201–205. doi: 10.1002/syn.890140303. [DOI] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28:2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, Zhou QG, Wu DL, Zhu LJ, Zhu DY. Neuronal nitric oxide synthase alteration accounts for the role of 5- HT1A receptor in modulating anxiety-related behaviors. The Journal of Neuroscience. 2010;30:2433–2441. doi: 10.1523/JNEUROSCI.5880-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]