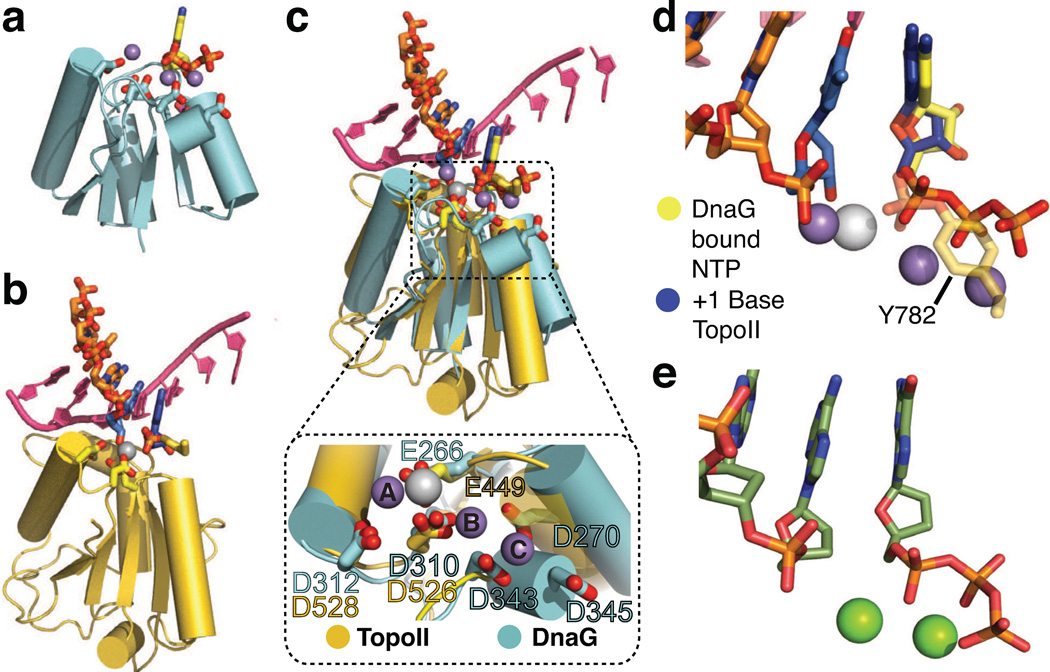
Figure 3. Alignment of nucleotide-bound structures with S. cerevisiae topoisomerase II.
a) Cartoon representation of the SaDnaG RPD TOPRIM fold bound to nucleotide (CTP). Sidechains of the conserved acidic residues in the DnaG TOPRIM fold are shown as sticks with carbon colored cyan and oxygen red. CTP and its associated Mn2+ ions are colored as in Fig 2a.
b) Cartoon representation of the S. cerevisiae topo II TOPRIM fold as found in a DNA-cleavage complex (PDB ID 3L4K). Sidechains of the conserved acidic residues and the covalently attached catalytic tyrosine (Tyr782) from the neighboring protomer are shown as sticks with carbon colored gold and oxygen in red. The scissile strand is shown as orange sticks. The −1 and +1 nucleobases at the cleavage site are colored blue. The complementary strand is shown in bright pink as a cartoon representation. The metal ion bound to the topo II catalytic center (Zn2+, in this particular complex) is shown as a white sphere.
c) Superposition of TOPRIM folds from CTP-bound SaDnaG RPD and the yeast topo II•DNA cleavage complex. The DnaG and topo II TOPRIM folds and sidechains, along with their associated substrates, are depicted as per panels (a) and (b), respectively. Boxed panel: close-up of the aligned metal binding regions (region highlighted by dashed outline in main panel) of both TOPRIM folds, with conserved acidic residues shown as stick representations and colored as per panels (a) and (b). The metal ions bound to topo II and SaDnaG are colored white and gray, respectively.
d) Close-up of substrate configuration based on a TOPRIM-fold alignment between DnaG and yeast topo II. Only the substrates from both structures are shown, and are colored as in panel (c). The covalent linkage between Tyr782 and DNA observed in topo II is shown as a semi-transparent stick representation.
e) Schematic of the active site in the T7 DNA polymerase ternary complex structure (PDB ID: 1T7P, (Doublie et al., 1998)). The incoming nucleotide and primer strand are shown in stick representations with carbon colored dark green. Mg2+ ions are shown as green spheres.
See also Figure S3.