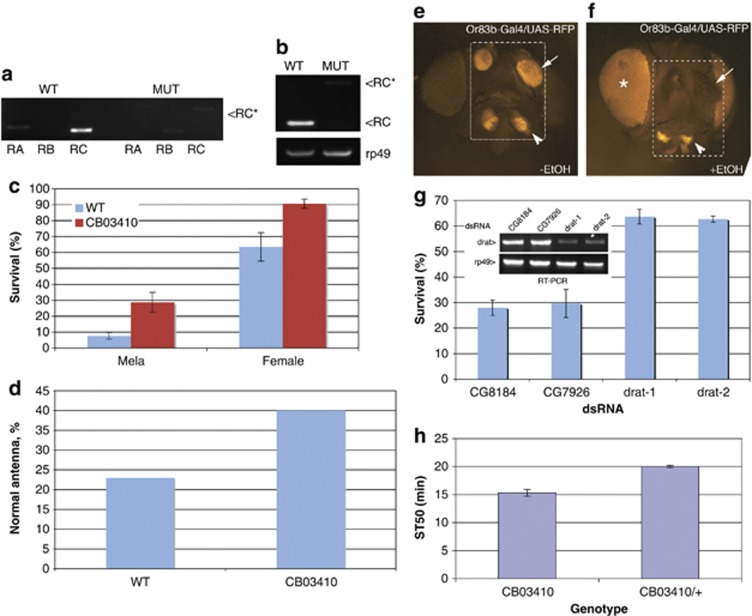
Figure 6.
Drat is an effector of alcohol-induced cell death in vivo. (a) RT-PCR of wild-type and P{PTT-GB}CG1600CB03410-mutant adults. Primers that are specific for each transcript of Drat (CG1600) were used to detect levels of Drat-RA, -RB and -RC. Note that in P{PTT-GB}CG1600CB03410, RA and RC fragments are larger than their wild-type counterparts by virtue of a transposon insertion. As the RB-specific primer spans the wild-type splicing junction, it failed to detect a GFP fusion transcript in P{PTT-GB}CG1600CB03410. The RC-GFP fragment (RC*) was sequenced to confirm the GFP insertion. (b) RT-PCR of homozygous P{PTT-GB}CG1600CB03410 and wild-type adults using primer pairs diagnostic for the RB and RC transcript isoforms in wild-type and CB03410 flies. RC* denotes Drat-RC-GFP fragment. (c) Three-day-old P{PTT-GB}CG1600CB03410 and wild-type adult flies were treated with ethanol vapor for 1 h, and survival was assessed after 24 h (see Materials and Methods). Data shown are the averages from five experiments, using a total of more than 700 adults. Error bars indicate standard deviation. (d) Three-day-old P{PTT-GB}CG1600CB03410 and wild-type adult flies were treated with ethanol vapor for 30 min and blackening of the third antennal segment was quantified the next day as in French and Heberlien.36 Data shown are averages from two independent trials. (e and f) Images of heads from control and ethanol-challenged Or83b-Gal4/UAS-RFP flies. The third segment of antenna (arrows) and maxillary palps (arrowheads) were labeled with RFP and killing of olfactory neurons by ethanol exposure was assessed by following loss of RFP in the third antennal segment as in French and Heberlien.36 The dotted boxes highlight RFP signal in the antenna and maxillary palps. Note that (f) is overexposed to appreciate RFP loss, resulting in nonspecific signal from the eyes indicated by *. (g) Note that ethanol-induced death of olfactory neurons cells is partially rescued by silencing of Drat. Flies expressing RFP and indicated dsRNAs under the control of Or83b-GAL4 were exposed to ethanol for 30 min, and the percentage of unaffected RFP-positive antenna (arrows) was scored the next day (mean±S.D., n=4). Inset: RT-PCR from flies expressing various dsRNA constructs driven by da-GAL4 confirms effective silencing of Drat by the relevant transgenes. In these experiments, two distinct Drat dsRNA transgenes (drat-1 and drat-2) and dsRNAs against two irrelevant genes CG8184 and CG7926 were used. (h) P{PTT-GB}CG1600CB03410 and P{PTT-GB}CG1600CB03410/+ flies were challenged with ethanol vapor, and median sedation time (ST50) was measured (mean±S.D., n=3; see Materials and Methods)