FIGURE 2.
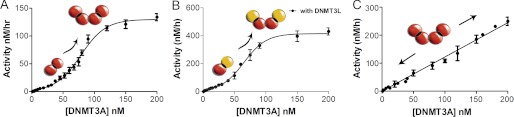
Concentration-dependent DNMT3A activation. A, the activity of DNMT3A shows a sigmoidal relationship with protein concentration. DNMT3A is more active at higher enzyme concentrations suggesting that DNMT3A oligomerization is concentration-dependent. The DNA is below the Km at 500 nm bp. B, DNMT3A with DNMT3L is more active at higher DNMT3A concentrations (DNMT3L is kept constant at saturating concentrations), suggesting that the concentration-dependent oligomerization takes place at the dimer interface (the interface that does not bind DNMT3L). The DNA is below the Km at 500 nm bp. C, the activity of DNMT3A shows a linear relationship when DNA is at over above the Km, data shown are when DNA is at 2 μm bp. Reactions took place on poly-dIdC, a multiple site substrate.
