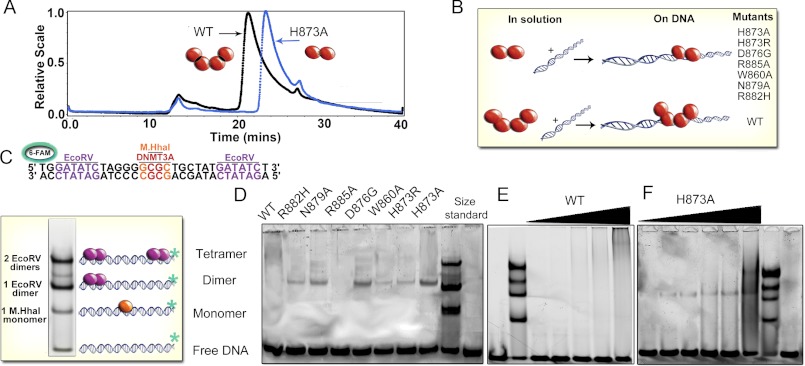
FIGURE 3.
Dimer interface mutants disrupt the interface resulting in dimers on DNA. A, size-exclusion chromatography of light scattering traces of tetrameric wild type catalytic domain (black trace) and representative dimeric H873A (blue trace). Molecular weights were determined from the amount of scattered light, in relation to protein concentration determined by A280. B, diagram of oligomeric mutants with and without DNA. C, EMSA of size markers, DNA (GCbox30) has binding sites for size standards, one site for M. HhaI (37 kDa), a known monomer and two binding sites for EcoRV (29 kDa), a known dimer, which creates dimer and tetramer bands. D, EMSA showed mutations at the dimer interface of DNMT3A disrupt oligomerization resulting in dimers on DNA (200 nm enzyme, 300 nm DNA). E, the wild type DNMT3A at varying concentrations (20–500 nm) migrates as a tetramer or larger on DNA (200 nm). F, the H873A mutant (20–500 nm) migrates as a dimer on DNA (200 nm) at varying concentrations.