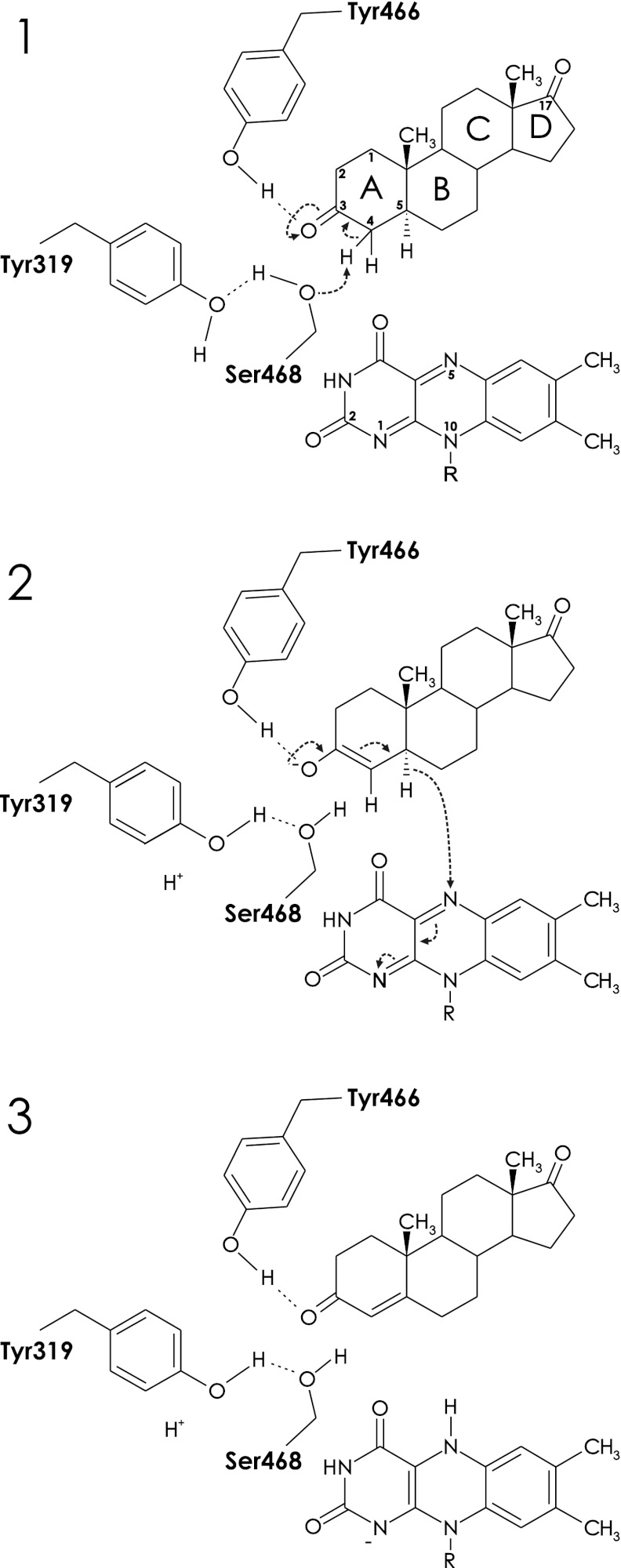
FIGURE 7.
Proposed reaction mechanism of Δ4-(5α)-KSTD. Panel 1, catalysis is initiated by the interaction of the O3 keto group of the substrate with the hydroxyl group of Tyr-466, which promotes keto-enol tautomerization and labilization of the C4 hydrogen atoms. Ser-468, acting as a base, abstracts a proton from the C4 atom of the substrate, with Tyr-319 serving as a proton relay system to the solvent. Panel 2, next, the FAD abstracts a hydride ion from the C5 carbon of the substrate, which, with a concomitant rearrangement, results in the formation of a double bond between C4 and C5. Panel 3, the product is formed and leaves the active site, perhaps only after the oxidizing substrate enters the active site. The negative charge on the reduced FAD is stabilized by the dipole moment of the C-terminal helix. Finally, the oxidized FAD is regenerated by an as yet unknown electron acceptor.