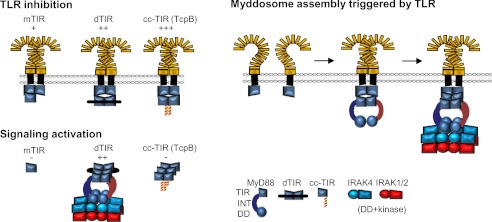
FIGURE 7.
Model of the role of dimeric TIR platform for the inhibition and activation of MyD88 signaling pathway. Left, monomeric and dimeric MyD88 TIR domains inhibit TLR signaling by binding to cytoplasmic TIR dimer of activated TLRs, preventing binding of MyD88. Tethered TIR dimer induce MyD88 oligomerization, which triggers assembly of the Myddosome complex. Right, model of the assembly of Myddosome induced by TLR dimerization: sequential addition of MyD88 through interactions with TIR of TLRs and TIRs of MyD88 assembles the DD complex and triggers phosphorylation of IRAK kinases.