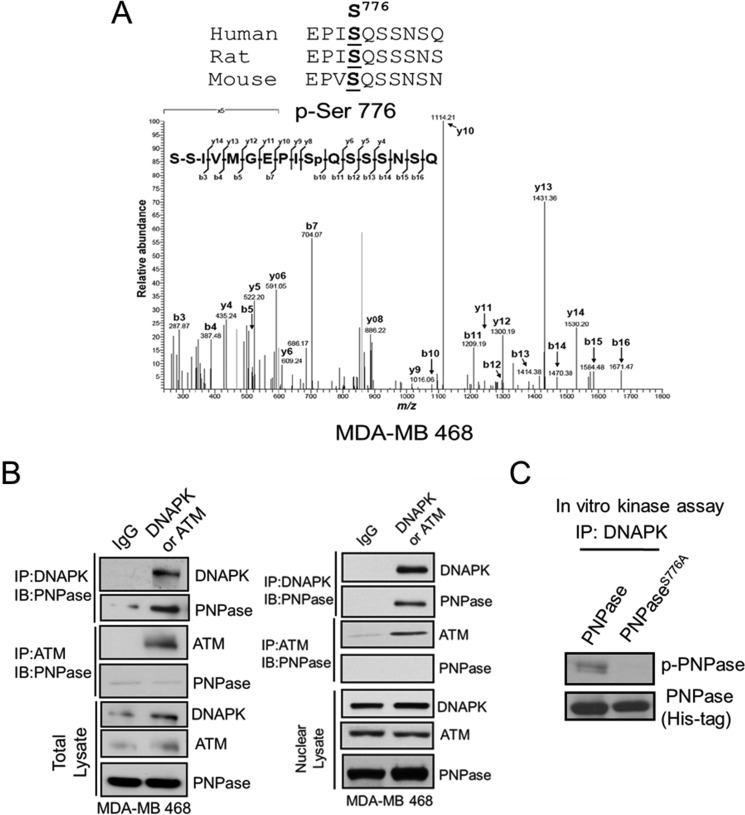
FIGURE 2.
Phosphorylation of PNPase at Ser-776 is mediated by DNAPK. A, PNPase in the nuclear lysate was isolated by IP from MDA-MB 468 cells. The phosphorylated residue of PNPase was identified at Ser-776 by tandem mass (MS/MS) spectrometric analysis. The top panel shows the alignment of the flanking regions of Ser-776 of PNPase among different species. B, total and nuclear lysates were immunoprecipitated with IgG, anti-DNAPK (top panel) or anti-ATM (middle panel) antibody, and PNPase in the IP complex was determined by IB. The expression of indicated protein in lysate was also examined by IB (bottom panel). C, purified recombinant His-tagged PNPase or PNPaseS776A proteins were incubated with immunoprecipitated DNAPK and applied to in vitro kinase assay. The reaction mixture was separated by SDS-PAGE, followed by IB with anti-p-Ser antibody (top). The Coomassie Blue-stained gel (bottom) served as loading control.