FIGURE 1.
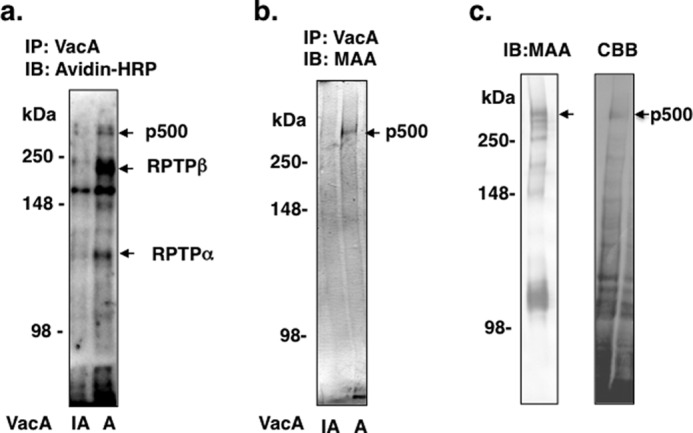
Purification of p500 from AZ-521 cells by MAA-agarose column. a, after biotinylation of surface proteins, AZ-521 cells were solubilized and immunoprecipitated with heat-inactivated (IA) or wild-type VacA (A) as described under “Experimental Procedures.” Immunocomplexes were separated by SDS-PAGE in 6% gels and transferred to PVDF membranes. VacA-binding proteins were detected with streptavidin-HRP. b, proteins immunoprecipitated (IP) with heat-inactivated or wild-type VacA were separated by SDS-PAGE in 6% gels and transferred to PVDF membranes, which were incubated with MAA-lectin conjugated to digoxigenin and then with anti-digoxigenin Fab fragments conjugated to alkaline phosphatase, followed by reaction with 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate. c, biotinylated AZ-521 cell lysates were incubated overnight with a MAA-agarose column (2 ml bed volume), which was washed with 20 ml of Sol buffer. Bound proteins were eluted, concentrated, and separated by SDS-PAGE as described under “Experimental Procedures.” MAA-lectin blotting is shown in the left panel and Coomassie Brilliant Blue (CBB) staining in the right panel. The stained p500 protein band was hydrolyzed with trypsin and subjected to LC-MS/MS analysis. The procedures described in a-c were repeated at least three times with similar results. IB, immunoblot.
