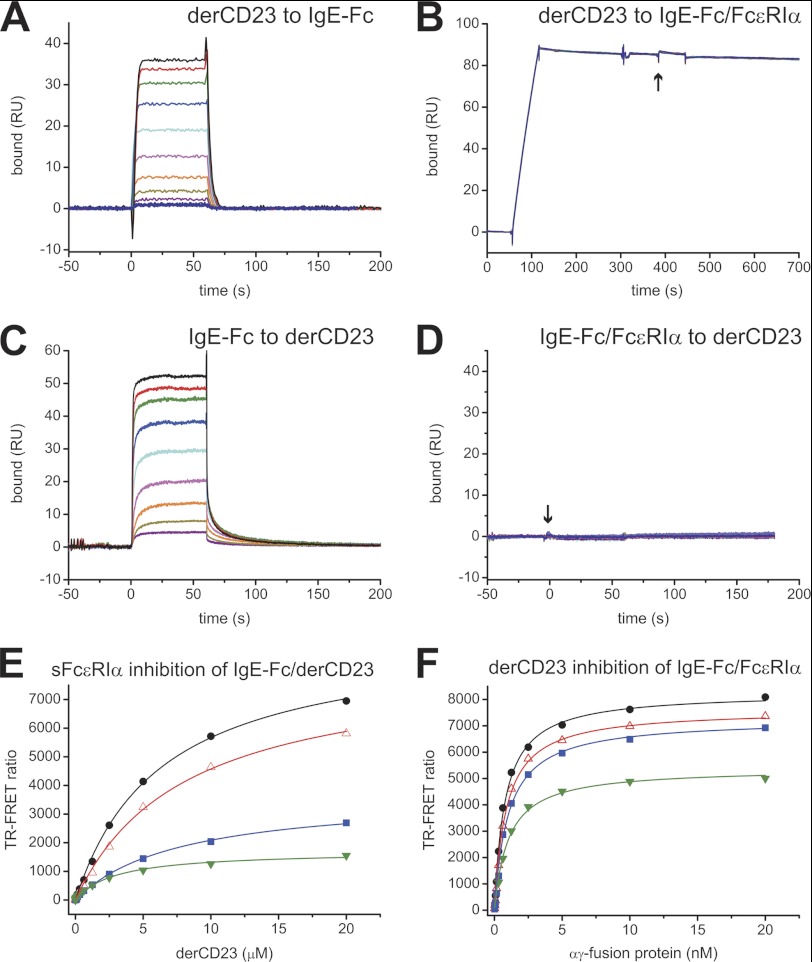
FIGURE 2.
Competition binding experiments between derCD23 and sFcϵRIα for IgE-Fc. A and B, the binding of derCD23 was tested against IgE-Fc immobilized on a sensor surface (A) and IgE-Fc captured on an FcϵRIα-immobilized surface (B); the start of the derCD23 injection is indicated with an arrow. DerCD23 bound to immobilized IgE-Fc with a KD of 2.3 μm; no measureable binding was observed for derCD23 to IgE-Fc complexed to FcϵRIα. RU, resonance units. C and D, the binding of IgE-Fc to immobilized derCD23 (C) was compared with the binding of a complex of IgE-Fc·sFcϵRIα (D) to the same surface; the start of the injection of the complex is indicated with an arrow. IgE-Fc bound to derCD23 with a KD of 2.4 μm, but the IgE-Fc·sFcϵRIα complex did not bind to derCD23. All SPR binding experiments were performed using identical 2-fold serial dilutions of ligands, from 40 μm to 78 nm. E, binding between terbium-labeled derCD23 and Alexa Fluor 647-labeled IgE-Fc was measured in a solution TR-FRET assay in the presence of increasing concentrations of unlabeled αγ-fusion protein as inhibitor: 0 nm (black), 0.5 nm (red), 2.5 nm (blue), and 5 nm (green). F, binding between terbium-labeled αγ-fusion protein and Alexa Fluor 647-labeled IgE-Fc was measured with increasing concentrations of unlabeled derCD23 as inhibitor: 0 μm (black), 25 μm (red), 50 μm (blue), and 185 μm (green).