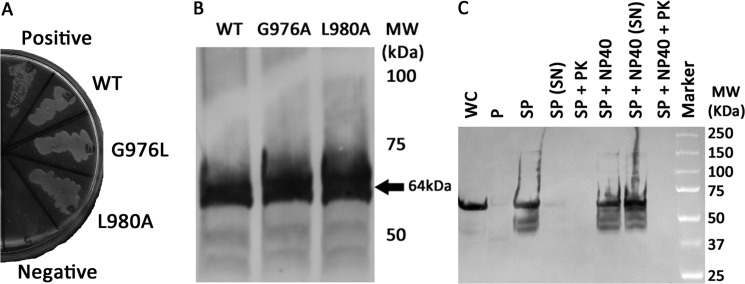
FIGURE 5.
The integrin αIIb TM-CYTO chimera express at similar levels and are properly integrated into the inner membrane of E. coli. A, AraTM chimeras containing wild-type and mutant integrin αIIb TM-CYTO expressed in MalE-deficient MM39 cells were streaked on a 0.4% maltose M9 plate and incubated for 48 h at 37 °C. Each construct is properly integrated into the inner membrane of E. coli, as indicated by robust growth on the 0.4% maltose M9 plates similar to the positive control (pTrcRSF containing MBP-AraC chimera). As expected, no growth is observed on the negative control (AraCY). B, wild-type and mutant integrin αIIb TM-CYTO chimera were expressed at equal levels as determined by immunoblotting with HRP-conjugated anti-MBP antibody, and the observed chimera MWs were consistent with the expected MWs. C, periplasts and spheroplasts were prepared for mutant integrin αIIb L980A TM-CYTO, treated with and without Nonidet P-40 (1% v/v) and proteinase K (50 μg/ml), and blotted against anti-MBP antibody (WC: whole cell, P: periplast, SP: spheroplast, SN: supernatant, PK: proteinase K, and Nonidet P-40: Detergent Nonidet P-40). No chimera is detected in intact spheroplasts treated with proteinase K (SP + PK) nor in the periplasmic fraction of the cell, consistent with the expected periplasmic orientation and membrane integration of the MBP fusion.