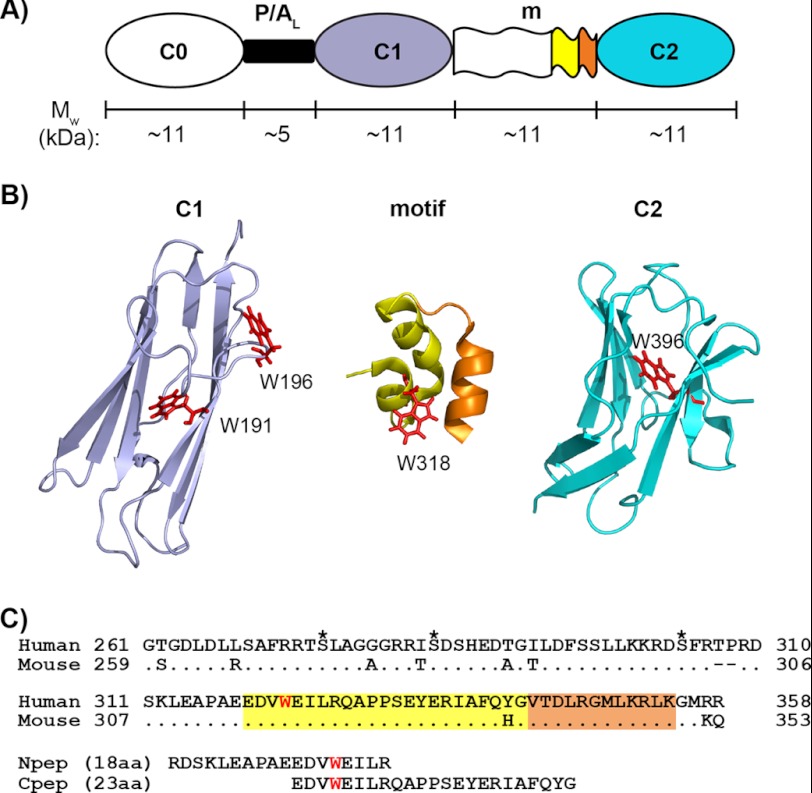
FIGURE 1.
N-terminal modules and the motif of cMyBP-C. A, schematic representation of the three N-terminal Ig modules (C0, C1, and C2) and their connectors: the proline/alanine-rich linker (P/AL) and the motif (m). The approximate molecular weight (Mw) of each region is indicated, and the color coding for different regions of sequence/structure is conserved in panels A, B, and C. B, ribbon diagrams of (from left to right) the human C1 (PDB 2AVG) module, the structured region of mouse motif (residues 315–351) (PDB 2LHU), and the human C2 (PDB 1PD6) module with tryptophan residues (Trp191, Trp196, Trp318, and Trp396) shown as red sticks. Note that Trp318 in the mouse sequence is equivalent to Trp322 in the human sequence. In the mouse motif ribbon diagram (middle), the sequence corresponding to human Cpep used in this study (incorporating Trp318) is shown in yellow, and the remaining sequence is orange. C, sequence alignments of human and mouse cMyBP-C motifs and of human motif peptides (Npep and Cpep). For the human motif, the three putative phosphorylation sites are marked by asterisks above the sequence, and the only tryptophan residue, Trp322, is highlighted in red. Sequences corresponding to the structured region of mouse motif (amino acids (aa) 315–351) are highlighted in yellow and orange as in B. Note that for the human triphosphorylation mimic C1C2EEE, the three putative phosphorylation sites are mutated to glutamates (i.e. S275E/S284E/S304E).