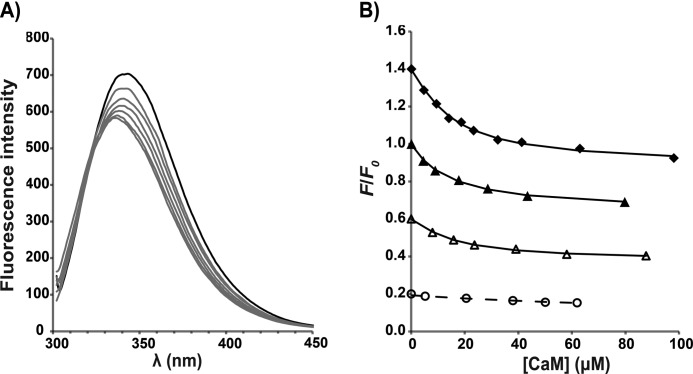
FIGURE 3.
Tryptophan fluorescence from the cMyBP-C N-terminal fragments C1C2 and C1C2EEE titrated with Ca2+/CaM. A, fluorescence emission spectra for the titration of C1C2 with CaM in the standard buffer and in the presence of Ca2+. A blue shift of the maximum emission wavelength is accompanied by a decrease in the maximum fluorescence intensity. The black line corresponds to the spectrum of the free protein before adding Ca2+/CaM, and the gray lines represent spectra from the sequential titration points. B, determination of binding affinities (KD) from different titration experiments. For each titration data set, the symbols represent the experimental data of the fluorescence intensity at each titration step (F) relative to the fluorescence in the absence of Ca2+/CaM (F0) at a chosen wavelength. The lines are fitted curves for determination of KD based on a 1:1 model. For clarity of representation, each titration data set has been scaled by multiplying with the following constants in the (F/F0) values: C1C2EEE, 1.4 (♦); C1C2, 1.0 (▴); C1C2 in 100 mm NaCl, 0.6 (△); and C1C2 in EGTA, 0.2 (○). Each data point is the average of eight replicate measurements after correcting for solvent; the error bars (not shown; corresponding to ±1 S.D.) are smaller than the symbols. Note that “C1C2 in 100 mm NaCl” data were acquired in buffer containing 100 mm NaCl, and “C1C2 in EGTA” data were acquired in buffer containing 10 mm EGTA; the rest of the titrations were performed in the standard buffer containing 350 mm NaCl (for details, see “Experimental Procedures”).