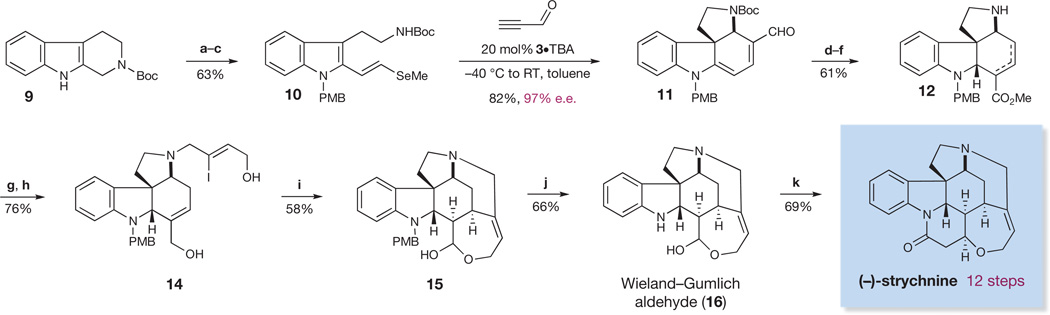
Figure 4. Twelve-step enantioselective total synthesis of (−)-strychnine.
Reagents and conditions are as follows. a, NaH, PMBCl, dimethylformamide (DMF), 0 °C. PMB, para-methoxybenzyl. b, SeO2, dioxane, H2O, 100 °C. c, (EtO)2P(O)CH2SeMe, 18-crown-6, potassium bis(trimethylsilyl)amide (KHMDS), tetrahydrofuran (THF), −78 °C to room temperature (RT, 23 °C). e.e., enantiomeric excess. d, (Ph3P)3RhCl, toluene, PhCN, 120 °C. e, COCl2, Et3N, toluene, −45 °C to RT, then MeOH,−30 °C to RT. f, DIBAL-H,CH2Cl2, −78 °C to RT, then trifluoroacetic acid (TFA), −78 °C to RT. g, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), K2CO3, DMF, (Z)-4-bromo-3-iodobut-2-enyl acetate (13), RT. h, DIBAL-H, CH2Cl2, −78 °C. i, 25 mol% Pd(OAc)2, Bu4NCl, NaHCO3, EtOAc, RT. j, PhSH, TFA, 45 °C. k, NaOAc, Ac2O, AcOH, malonic acid, 120 °C. Ac, acetyl.