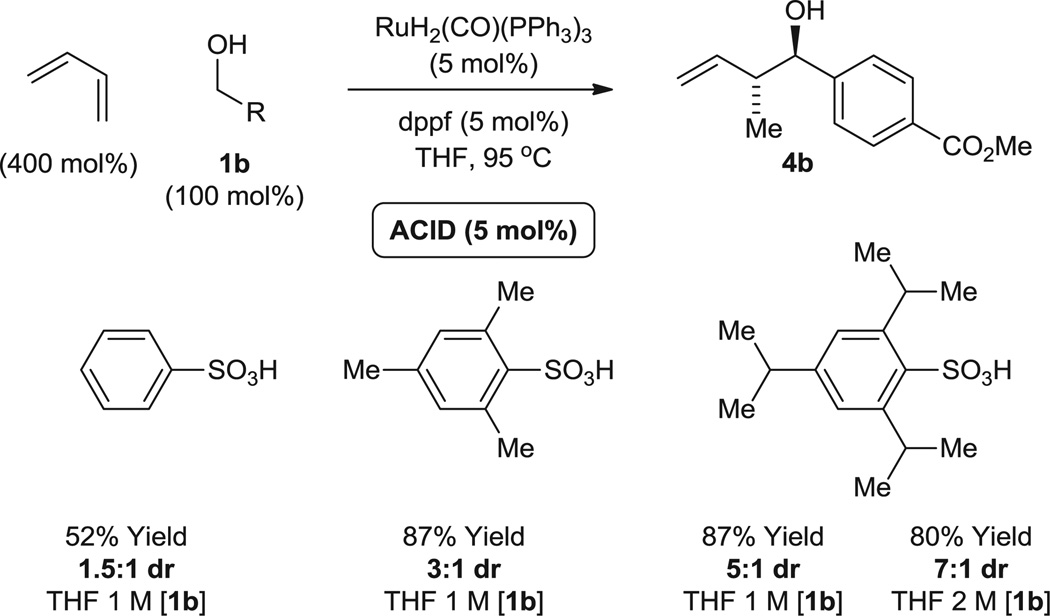
Fig. 2.
Enhanced anti-diastereoselectivity in response to increasing steric demand of the ruthenium counterion in the hydrohydroxyalkylation of benzylic alcohol 1b. Yields are of material isolated by silica gel chromatography. Diastereomeric ratios were determined by 1H NMR analysis of crude reaction mixtures. See the supplementary materials for further details.