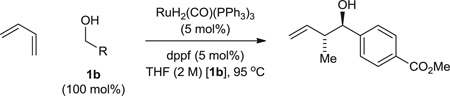
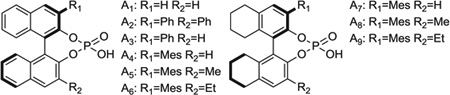
Table 1.
Optimization of anti-diastereoselectivity and enantioselectivity in the hydrohydroxyalkylation of benzylic alcohol 1b using BINOL-derived phosphate counterions. Yields are of material isolated by silica gel chromatography. Diastereomeric ratios were determined by 1H NMR analysis of crude reaction mixtures. ERs (er) were determined by chiral stationary-phase high-performance liquid chromatography analysis. These data represent only a small sampling of conditions and phosphoric acids that were screened. For additional details, see supplementary materials text.
 | |||||
|---|---|---|---|---|---|
| Entry | Acid (mol%) | Butadiene | Time (hrs) | Yield% | er (dr) |
| 1 | A1 (5) | 400 mol% | 19 | 45 | 52:48 (1:1) |
| 2 | A2 (5) | 400 mol% | 19 | 91 | 57:43 (5:1) |
| 3 | A3 (5) | 400 mol% | 19 | 83 | 69:31 (2:1) |
| 4 | A4 (5) | 400 mol% | 19 | 84 | 87:13 (3:1) |
| 5 | A4 (5) | 100 mol% | 19 | 72 | 87:13 (3:1) |
| 6 | A4 (5) | 200 mol% | 19 | 80 | 87:13 (3:1) |
| 7 | A4 (5) | 800 mol% | 19 | 50 | 87:13 (3:1) |
| 8 | A5 (5) | 400 mol% | 19 | 15 | 85:15 (6:1) |
| 9 | A6 (5) | 400 mol% | 19 | 21 | 87:15 (5:1) |
| 10 | A7 (5) | 400 mol% | 19 | 45 | 89:11 (3:1) |
| 11 | A8 (5) | 400 mol% | 19 | 15 | 86:14 (5:1) |
| 12 | A9 (5) | 400 mol% | 19 | 29 | 86:14 (4:1) |
| 13 | A6 (10) | 400 mol% | 19 | 33 | 92:8 (7:1) |
| 14 | A7 (5) | 400 mol% | 19 | 48 | 90:10 (3:1) |
| 15 | A9 (10) | 400 mol% | 19 | 50 | 94:6 (7:1) |
| ⇨16 | A9 (10) | 400 mol% | 48 | 80 | 94:6 (7:1) |
 | |||||
