Abstract
Background
A variety of indicators of potentially successful ovarian stimulation cycles are available, including biomarkers such as anti-Mullerian hormone. The aim of our study was to confirm the usefulness of serum anti-Mullerian hormone assay in predicting ovarian response and reproductive outcome in women eligible for ART cycles.
Materials
Forty-six women undergoing ART cycles at the Centre for Reproductive Medicine in Parma were recruited from March-to-June 2010. Inclusion criteria: age<42 years; body-mass-index = 20–25; regular menstrual cycles; basal serum FSH concentration <12 IU/L and basal serum estradiol concentration <70 pg/mL. The couples included in our study reported a variety of primary infertility causes. All women underwent FSH stimulation and pituitary suppression (GnRH-agonist/GnRH-antagonist protocols). Women were considered poor-responders if thay had ≤3 oocytes; normal-responders 4–9 oocytes and high-responders ≥10 oocytes. Serum samples for the AMH assays were obtained on the first and last days of stimulation. A P value ≤0.05 was considered statistically significant.
Result
FSH levels increased significantly when AMH levels decreased. The total dose of r-FSH administered to induce ovulation was not correlated to AMH. The number of follicles on the hCG, serum estradiol levels on the hCG-day, and the number of retrieved oocytes were significantly correlated to AMH. The number of fertilized oocytes was significantly correlated to the AMH levels. No significant correlation was found between obtained embryos or transferred embryos and AMH. Basal serum AMH levels were significantly higher than those measured on the hCG-day, which appeared significantly reduced. There was a significant correlation between AMH in normal responders and AMH in both high and poor responders.
Conclusions
Our data confirm the clinical usefulness of AMH in ART-cycles to customize treatment protocols and suggest the necessity of verifying an eventual permanent decrease in AMH levels after IVF.
Introduction
Appropriate clinical evaluation and proper treatment of women are essential for a positiveoutcome of assisted reproductive technology (ART) cycles. For good results it is necessary to assess ovarian reserve before planning treatment. The identification of both low and high responders before treatment may reduce cycle cancellation rates and side-effects, such as ovarian hyperstimulation syndrome (OHSS) [1].
Biomarkers with well-understood biological mechanisms and metrics for assay interpretation are needed to provide an ovarian frame for the onset and the end of the menopause transition, as well as to indicate the proximity to the final menstrual period, and to contribute to clinical decision making [2].
For several years, age and day-3 levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) have been used as indicators of ovarian response toART.In fact, the basal FSH concentration is the most common test used for ovarian screening [3], however, it has been reported that the increase in FSH levels occurs late in the sequence of events associated with ovarian aging [4]. Therefore, if fertility is considered the end point, this increase may be of limited clinical use as a marker [5]. Recently, several investigators reported the effectiveness of antral follicle count (AFC) and ovarian volume in predicting ovarian response to hormonal stimulation [6], [7]. They stated that AFC provides better prognostic information on the occurrence of poor ovarian response during hormone stimulation for in vitro fertilization (IVF) than does the woman’s chronological age or basal FSH. Nonetheless, ultrasoundis subjective, and the interpretation of the observations may not be consistent [8]. So the need persists for a biological endocrine marker that can be used without bias.
Recently, a new endocrine marker, anti-Müllerian hormone (AMH), was evaluated by several study groups as a marker of ovarian response.
AMH is a dimeric glycoprotein member of the transforming growth factor (TGF)-β superfamily. Its most clearly defined role is in male sexual differentiation. AMH is produced by fetal Sertoli cells at the time of testicular differentiation, and induces regression of the Müllerian ducts. In the absence of AMH, the Müllerian ducts develop into the uterus, fallopian tubes and the upper part of the vagina [9]. In women, AMH is produced in the ovary by the granulosa cells surrounding preantral and small antral follicles [10], [11]. AMH expression in ovaries has been observed as early as 36 weeks gestation in humans [12]. Even when using ultrasensitive assays, AMH is barely detectable in the serum at birth. Later, AMH increases after puberty [12], [13] and then declines with advancing female age, to become undetectable again at the time of the menopause [14].
AMH levels correlate well with the number of antral follicles measured by ultrasound [15]–[17] and are believed to be the best representation of the gradual decline in reproductive capacity in women proven to be fertile [18], [19]. Finally, AMH has been shown to be an accurate marker for the occurrence of poor response to ovarian hyperstimulation with gonadotropins in IVF [16], [20], [21]. Based on these findings, AMH may well become a frequently applied marker in reproductive medicine [22].
The purpose of our study was to confirm the usefulness of the serum AMH assay in predicting ovarian response and reproductive outcome in women undergoing ovarian stimulation in ART cycles.
Methods
For our study we recruited 46 women undergoing ART cycles at the Centre for Reproductive Medicine of the University of Parma - Department of Obstetrics, Gynecology and Neonatology. The study was conducted from 1st March 2010 to 30th June 2010.
All women gave their written informed consent before receiving medical treatment and the study was approved by the University of Parma Ethics Board. Inclusion criteria were: age <42 years; body mass index 20–25; regular menstrual cycles lasting between 26 and 34 days; basal serum FSH concentration (on day 3 of the menstrual cycle) <12 IU/L; basal serum estradiol concentration <70 pg/mL.
The couples included in our study reported a variety of primary infertility causes, including male factors, tubal factors, endometriosis, and idiopathic causes. Ultrasound examinations revealed uteruses and ovaries of normal size and shape. Semen parameters were evaluated according to the World Health Organization guidelines [23].
All women underwent FSH stimulation and pituitary suppression with an agonist or antagonist of the gonadotropin-releasing hormone (GnRH). The GnRH agonist (leuprolide acetate 0.25 mg/day s.c.) was administered starting from the mid-luteal phase (day 21) of the previous menstrual cycle. After pituitary desensitization, the women were stimulated with recombinant FSH at doses of 225–450 IU/day s.c. The GnRH antagonist (ganirelix acetate 0.25 mg/day s.c.) was administered only after obtaining at least one ≥14 mm follicle in women who had been previously treated from day 3 of the menstrual cycle with recombinant FSH at doses of 225–450 IU/day s.c. FSH was withdrawn after follicular maturation was achieved. Follicular maturation parameters were assessed daily by transvaginal ultrasound (using a Philips HD3 ultrasound system with 6.5-MHz transducer) and serum estradiol assays. As soon as follicular maturation was achieved (with follicles 16–18 mm in diameter and a serum estradiol concentration >1,000 pg/mL), women received 10,000 IU of human chorionic gonadotropin (hCG). Oocytes were retrieved after 34–36 hours by ultrasound-guided transvaginal follicular aspiration using a 17-gauge needle. Oocyte pick-up was carried out under general anesthesia.
As protection in the luteal phase, women were given 200 mg of micronized progesterone vaginally, twice a day, starting from the day before embryo transfer (42–72 hours after oocyte pick-up). Women were considered poor responders when ≤3 oocytes were retrieved or when the ART cycle was cancelled because there was no follicular growth following controlled stimulation. Normal responders had 4–9 oocytes retrieved, while high responders had ≥10 oocytes retrieved or the women had OHSS. Serum samples for the AMH assay were obtained on the first day of stimulation (at baseline) and on the last day of stimulation (the day of hCG administration). Suitable samples were centrifuged for 10 minutes at 2,000 revs and then frozen at −20°C until the AMH assay was performed. AMH was assayed in duplicate using an immunoenzymatic technique (Immunotech, Marseille, France). Assay sensitivity was 0.14 ng/mL; intra- and inter-assay coefficients of variation were ≤12.3% and ≤14.2%, respectively.
Serum estradiol concentrations were assayed in duplicate using a radioimmunological technique with the Coat-A-Count E2 kit (Diagnostic Products Corporation, Los Angeles, CA, USA).
Our data were expressed as means and standard deviations or as frequency rates. Depending on the type of data considered, the statistical analysis was carried out using descriptive statistical methods, Pearson’s correlation test, confidence interval calculation, Student’s t-test for paired data, Spearman’s correlation test and the Mann-Whitney U-test. A P value of ≤0.05 was considered statistically significant.
Results
Pre-treatment characteristics and demographic data of the women studiedare reported in Table 1a . Etiological factors of infertility included male factors (28 cases), tubal factors (9 cases), endometriosis (2 cases), and idiopathic factors (7 cases). Mean AMH values for different etiological factors of infertiulity are reported in Table 1b .
Table 1a. a ,b. Pre-treatment features, patient demographic data and mean AMH levels for different etiological factors of infertility.
| aPre-treatment features and demographic data | N° patients | Mean | DS |
| Age (years) | 46 | 35,50 | 4,09 |
| BMI (kg/m2) | 46 | 22,21 | 2,39 |
| basal FSH (UI/l) | 46 | 8,18 | 2,64 |
| basal LH (UI/l) | 46 | 4,74 | 2,02 |
| basal Estradiol (pg/ml) | 46 | 52,40 | 17,40 |
| basal AMH (ng/ml) | 46 | 4,02 | 4,36 |
| bEtiological factors of infertilità | N° patients | Mean AMH levels | |
| Male factors | 28 | 4.06 | |
| Tubal factors | 9 | 4.3 | |
| Endometriosis | 2 | 3.4 | |
| Idiopathic factors | 7 | 3.7 | |
The ART cycle was cancelled in two patients (4.35%) due to absent follicular growth. Embryo transfer was performed in 41 patients (89.13%). Data about ovarian response and reproductive outcome during controlled ovarian stimulation are reported in Table 2.
Table 2. Ovarian response and reproductive outcome during ovarian stimulation.
| N° patients | Mean | DS | |
| AMH on day of HCG (ng/ml) | 44 | 1,19 | 0,82 |
| Days of stimulation | 44 | 9,57 | 2,76 |
| Total dose of administered FSH (IU) | 44 | 2298,30 | 931,11 |
| Estradiol on day of HCG (pg/ml) | 44 | 1798,30 | 1068,52 |
| Follicles on day of HCG | 44 | 10,91 | 5,93 |
| Collected oocytes | 41 | 5,07 | 2,742 |
| Fertilized oocytes | 41 | 3,41 | 2,27 |
| Obtained embryos | 41 | 2,29 | 1,65 |
| Transferred embryos | 41 | 2,10 | 1,41 |
No significant correlation was found between basal AMH and a woman’s age, basal LH and basal estradiol levels (p: n.s.); while the correlation between basal AMH and basal FSH levels is statistically significant (p: 0.007). In fact, FSH levels (8.78±4.04) increased significantly when AMH levels decreased (ρ<0; P<0.01) (Table 3a ).
Table 3. Correlations between AMH serum level and pre/post treatment features.
| N° patients | ρ | p | |
| aAge | 46 | −0,224 | 0,134 |
| aBasal LH | 46 | −0,038 | 0,808 |
| aBasal estradiol | 46 | −0,093 | 0,623 |
| a Basal FSH | 46 | −0,400 | 0,007 |
| bTotal dose of administered FSH (IU) | 44 | −0,285 | 0,064 |
| c Follicles on day of HCG | 44 | 0,662 | <0,001 |
| c Estradiol on day of HCG | 44 | 0,548 | <0,001 |
| c Collected oocytes | 41 | 0,643 | <0,001 |
| d Fertilized oocytes | 41 | 0,400 | 0,009 |
| dObtained embryos | 41 | 0,294 | 0,062 |
| dTransferred embryos | 41 | 0,289 | 0,067 |
Notes:
Correlation between AMH and pre-treatment features of patients;
Correlation between AMH and total dose of administered FSH;
Correlation between AMH and ovarian response;
Correlation between AMH and reproductive outcome.
The total dose of administered recombinant FSH to induce ovulation (2,298±931.11) was not correlated to AMH (p: n.s.) (Table 3b ). Ovarian response, distinguished by the number of follicles on the hCG day (10.91±5.93), serum estradiol levels on the hCG day (1,798.30±1,068.52), and the number of retrieved oocytes (5.07±2.74) was significantly correlated to AMH (ρ >0; P<0.01) (Table 3c ). The number of fertilized oocytes (3.41±2.27) was also significantly correlated to AMH (ρ>0; P<0.01). No significant correlation was found between obtained embryos (2.9±1.65) or transferred embryos (2.10±1.41), and AMH (P = n.s.) (Table 3d ).
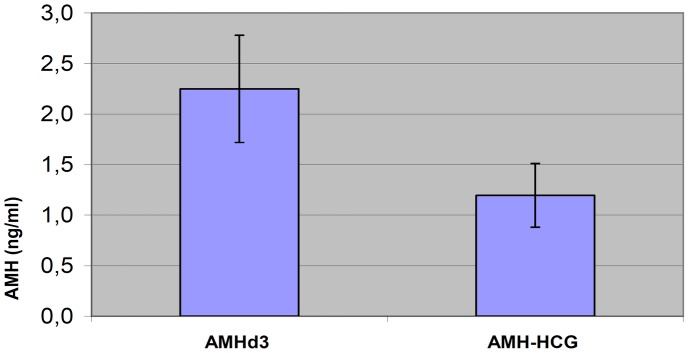
Basal serum AMH levels (on day 3 of the menstrual cycle) were significantly higher than AMH levels measured on the hCG day (p<0.01) (Fig. 1).
Figure 1. Values of serum AMH: baseline and on day of hCG administration level.
Notes: AMHd3 = AMH on the 3th day of the menstrual cycle (baseline). AMH-HCG = AMH on the day of hCG administration. T test for paired data. AMHd3 vs AMH-HCG: t = 5,484; p<0,001.
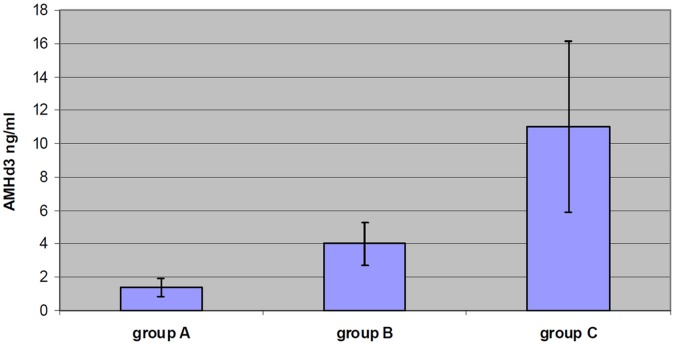
The AMH values in the different groups of responders to ovarian stimulation are reported in Table 4. There was a significant correlation between AMH in normal responders and AMH in both high and poor responders (Fig. 2). The correlation of AMH between poor and high responders was also significant as reported in Table 5.
Table 4. AMH levels in different response groups to ovarian stimulation.
| Poor responders(≤3 obtained oocytes/cancellationof the cycle) | Normal responders(4–9 obtained oocytes) | High responders(≥10 obtained oocytes/OHSS) | |
| N° patients | 15 | 25 | 6 |
| Mean | 1,4 | 4 | 11 |
| DS | 1,1 | 3,2 | 6,4 |
| Minimum | 0 | 0,91 | 2,58 |
| Maximum | 3,45 | 14,37 | 21 |
| Median | 1,25 | 3,09 | 11,01 |
Notes: OHSS = ovarian hyperstimulation syndrome.
Figure 2. values of AMHd3 and their 95% confidence intervals according to response group.
Notes: group A = poor responders; group B = normal responders; group C = high responders; AMH group B vs AMH group A: P<0,001; AMH group B vs AMH group C: P<0,001.
Table 5. Mann-Whitney test for comparison between poor responders and high responders.
| Z | P | |
| AMH group A vs. AMH group C | −3,23 | <0,001 |
Notes: group A = poor responders; group C = high responders.
Discussion
Our study confirmed the usefulness of AMH as a biomarker of ovarian function. We showed an inverse correlation between serum AMH and serum FSH levels measured on the third day of the menstrual cycle. We also noted that high levels of AMH were positively correlated with the number of retrieved and fertilized oocytes, which are all indicators of ovarian function.
Many other studies suggest AMH as a novel measure of ovarian reserve. AMH levels decrease throughout a woman’s reproductive life [15], [24]. Serum levels on day 3 of the menstrual cycle show a progressive decrease with age, which correlates with AFC [15]. Undetectable AMH levels after spontaneous menopause have been reported [14], [18], [24]. Ovariectomy in regularly cycling women is associated with the disappearance of AMH in 3–5 days, demonstrating that circulating AMH is exclusively of ovarian origin [14], [24]–[26]. AMH is an endocrine marker that reflects the transition of resting primordial follicles to growing follicles. AMH declines gradually in the 5 years prior to the final menstrual period, perhaps representing a critical biological juncture during the transition to menopause [2].
Once again, AMH is the best indicator of ovarian health, and therefore it is a crucial parameter for IVF success.
In Western societies, the introduction in the 1960s of reliable methods of contraception has led to the birth of fewer children per family. Driven by increasing levels of female education, a growing participation in the labour force and career demands, postponement of childbearing has been a secondary consequence of the so-called sexual revolution [27]. These societal changes in family planning have caused a significant increase in the incidence of unwanted infertility due to female reproductive ageing [27]–[30]. The reduction in female fertility has also be shown in contemporary population studies. The chance of not conceiving a first child within one year increases from under 5% in women in their early 20 s to approximately 30% or more in women aged 35 and older29. So, although the majority of older women will become pregnant within a one-year period, the chance of becoming subfertile increases about sixfold in comparison with very young women [27].
Precisely for these reasons, the use of IVF has grown exponentially in Western countries. Nonetheless, the probability of live births obtained through IVF treatment clearly decreases after age 35 [31].
The same is true for the implantation rate per embryo [32]. In fact, female age has consistently been shown to be an important predictor of successful IVF treatments [27].
Generalizing in medicine is incorrect since we all know that biological parameters vary widely from individual to individual. For this reason, we tried to find a biological parameter that would allow gynecologists to better identify patients’ ovarian quality in order to choose the most appropriate drug treatments. This biomarker appears to be AMH.
The parameters we studied to investigate the correlation between AMH and ovarian response were follicle count and estradiol concentration per day of hCG, and the number of retrieved oocytes. We found a significant correlation between AMH and these parameters.
The AMH value as a predictor of ovarian response in controlled ovarian stimulation cycles was confirmed in several studies [1], [15]–[21], which demonstrated a significant correlation both with the number of follicles on the hCG day [1], [33] and with the number of retrieved oocytes [16], [34], [35].
Granulosa cells of primary follicles show homogeneous AMH expression; in larger follicles, AMH is mainly produced in cells near the oocyte and in a few cells surrounding the antrum. AMH continues to be expressed in growing follicles in the ovary until they have reached adequate size and state of differentiation to be selected for dominance by the action of pituitary FSH. In the mouse this occurs at the early antral stage in small growing follicles [9], while in women in antral follicles of size 4–6 mm [11], [12]. AMH is not expressed in atretic follicles and theca cells [11], [12], [24], [36]–[39].
AMH levels therefore represent a useful indicator of the number of follicles in the early stage, which are transformed into larger follicles during controlled ovarian stimulation as a result of exogenous FSH administration [17].
In our study we found a significant correlation between AMH values and responder groups (poor responders, normal responders and high responders). AMH values were higher in high responders and lower in poor responders. Knowing the baseline values of AMH allowed us to choose the amount of FSH to be administered to each patient, thus saving on government spending and getting the best response from women. For example, a young woman with diminished ovarian reserve (based on AMH values) may be given preference on the waiting lists of public treatment centres (ranging from 6 to 12 months) to start of the first IVF cycle and the IVF specialist may choose for this woman a stronger-than-usual stimulation treatment.
OHSS seems to be associated with significantly higher basal AMH levels [1], [40], [41]. This suggests its clinical usefulness in preventing an excessive response.
In our study, AMH did not appear to influence reproductive outcome. However, this finding does not seem important because our protocols were not affected by basal AMH values, as we chose to assess them only after embryo transfer. This must make us reflect on whether we should use customized protocols to improve pregnancy rates.
Another finding of our study, which is somewhat at variance with the literature but is nonetheless extremely important, is the inverse relationship between basal AMH and the hCG day. As the AMH measurement was done before administering hCG, the unexpected results cannot be attributed to massive luteinization of the follicles, which might explain the fact that serum AMH levels do not significantly change throughout the menstrual cycle [42].
By contrast, in our study, baseline AMH values were significantly higher than those of AMH on the hCG day. The initial purpose of our measurement was to confirm this fact. What we found instead were unexpected results, which we will continue to assess in other patients and hopefully will be able to publish soon. Such results are not easily explained.
A few authors believe that AMH levels gradually decrease during ovarian stimulation due to a strong decrease in the number of small antral follicles, associated with the progressive increase in the number of larger follicles [43]. Several studies conducted in rats [39], [44] and on the ovarian tissue of adult women [11] showed decreased expression levels of AMH in larger antral follicles compared to smaller ones. Albeit valid, this theory cannot explain the significant decrease that we found in AMH levels. Such a significant reduction cannot be related to an increase in preovulatory follicles, especially because prenatal follicles continue to be expressed, sometimes in large numbers.
A recent study showed that the highest level of AMH expression was found in the granulosa cells of secondary, preantral and small antral follicles <4 mm in diameter. In larger (4–8 mm) antral follicles, AMH expression gradually disappeared [11]. Moreover, early follicle growth in humans appears to be independent of stimulation by gonadotropins [45].
We are currently conducting a prospective trial on the advisability to reconsider AMH concentrations after about 60 days to determine if the decrease in hormone levels reduction is transient or not. In the case that it is intransient, with each IVF cycle we should see a dramatic reduction of ovarian reserve and we should reconsider the maximum number of stimulations to prevent premature menopause.
The reduction in AMH levels observed during FSH administration may be due to a negative role of FSH on AMH secretion. Indeed, it is well established that FSH is a positive regulator of testicular AMH gene expression [46], whereas other Authors [39] previously reported that FSH may down-regulate AMH and AMH type-II receptor (AMHRII) expression in adult rat ovaries. Alternatively, the reduction in AMH levels could be due to the supraphysiological increase in estradiol levels observed when exogenous FSH is administered. Estradiol has been implicated in the down-regulation of AMH and AMHRII mRNA in the ovary [24], [39]. Therefore, this finding is not clear and needs to be confirmed by further studies.
In conclusion, our study suggests that the clinical usefulness of AMH in ART cycles consists in the possibility of customizing treatment protocols. This would make it possible for patients to entertain more realistic expectations and minimize both the psychological stress related to poor response or cycle cancellation and OHSS-related morbidity.
Funding Statement
These authors have no support or funding to report.
References
- 1. La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, et al. (2007) Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod 22: 766–71. [DOI] [PubMed] [Google Scholar]
- 2. Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, et al. (2008) Mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 93: 3478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loverro G, Nappi L, Mei L, Giacomoantonio L, Carriero C, et al. (2003) Evaluation of functional ovarian reserve in 60 patients. Reprod Biomed Online 7: 200–4. [DOI] [PubMed] [Google Scholar]
- 4. Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, et al. (1996) Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab 81: 1038–45. [DOI] [PubMed] [Google Scholar]
- 5. Bancsi LF, Muijs AM, den Ouden CT, Broekmans FJ, Looman CW, et al. (2000) Basal follicle stimulating hormone levels are of limited value in predicting ongoing pregnancy rates after in vitro fertilization. Fertil Steril 73: 552–7. [DOI] [PubMed] [Google Scholar]
- 6. Bancsi LF, Brockmans F, Eijkemans M, de Jong F, Habbema D, et al. (2002) Predictors in poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril 77: 328–36. [DOI] [PubMed] [Google Scholar]
- 7. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH (1998) Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril 69: 505–10. [DOI] [PubMed] [Google Scholar]
- 8. Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M (2004) Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG 111: 1248–53. [DOI] [PubMed] [Google Scholar]
- 9. Munsterberg A, Lovell-Badge R (1991) Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development 113: 613–624. [DOI] [PubMed] [Google Scholar]
- 10. Durlinger AL, Visser JA, Themmen AP (2002) Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction 124: 601–9. [DOI] [PubMed] [Google Scholar]
- 11. Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, et al. (2004) Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10: 77–83. [DOI] [PubMed] [Google Scholar]
- 12. Rajpert E, Jorgensen N, Graem N, Muller J, Cate RL, et al. (1999) Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 84: 3836–44. [DOI] [PubMed] [Google Scholar]
- 13. Guibourdenche J, Lucidarme N, Chevenne D, Rigal O, Nicolas M, et al. (2003) Anti-Mullerian hormone levels in serum from human foetuses and children: pattern and clinical interest. Mol Cell Endocrinol 211: 55–63. [DOI] [PubMed] [Google Scholar]
- 14. La Marca A, De Leo V, Giulini S, Orvieto R, Malmusi S, et al. (2005) Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Invest 12: 545–8. [DOI] [PubMed] [Google Scholar]
- 15. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC (2002) Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 77: 357–62. [DOI] [PubMed] [Google Scholar]
- 16. van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, et al. (2002) Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 17: 3065–71. [DOI] [PubMed] [Google Scholar]
- 17. Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, et al. (2003) Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod 18: 323–7. [DOI] [PubMed] [Google Scholar]
- 18. van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, et al. (2004) Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause 11: 601–6. [DOI] [PubMed] [Google Scholar]
- 19. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, et al. (2005) Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83: 979–87. [DOI] [PubMed] [Google Scholar]
- 20. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, et al. (2004) Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril 82: 1323–9. [DOI] [PubMed] [Google Scholar]
- 21. Fanchin R, Méndez Lozano DH, Louafi N, Achour-Frydman N, Frydman R, et al. (2005) Dynamics of serum anti-Müllerian hormone levels during the luteal phase of controlled ovarian hyperstimulation. Hum Reprod 20: 747–51. [DOI] [PubMed] [Google Scholar]
- 22. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, et al. (2006) Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab 91: 4057–63. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. (2010) WHO laboratory manual for the examination and processing of human semen, fifth edition. World Health Organization, Geneva.
- 24. La Marca A, Volpe A (2006) Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 64: 603–10. [DOI] [PubMed] [Google Scholar]
- 25. Rey RA, Lhommé C, Marcillac I, Lahlou N, Duvillard P, et al. (1996) Antimüllerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol 174: 958–65. [DOI] [PubMed] [Google Scholar]
- 26. Long WQ, Ranchin V, Pautier P, Belville C, Denizot P, et al. (2000) Detection of minimal levels of serum anti-Müllerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. J Clin Endocrinol Metab 85: 540–4. [DOI] [PubMed] [Google Scholar]
- 27. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12: 685–718. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein M, Wood AJ and Chang MC (1993) Age patterns in fecundability. In Gray R, Leridon H and Spira A (eds) Biomedical and Demographic Determinants of Reproduction. Clarendon Press, Oxford, 209–220.
- 29. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ (1997) Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital Health Stat 19: 1–114. [PubMed] [Google Scholar]
- 30. Ventura SJ, Mosher WD, Curtin SC, Abma JC, Henshaw S (2001) Trends in pregnancy rates for the United States, 1976–97: an update. Natl Vital Stat Rep 49: 1–9. [PubMed] [Google Scholar]
- 31. Templeton A, Morris JK, Parslow W (1996) Factors that affect outcome of in-vitro fertilisation treatment. Lancet 348: 1402–6. [DOI] [PubMed] [Google Scholar]
- 32. van Kooij RJ, Looman CW, Habbema JD, Dorland M, te Velde ER (1996) Age-dependent decrease in embryo implantation rate after in vitro fertilization. Fertil Steril 66: 769–75. [DOI] [PubMed] [Google Scholar]
- 33. Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, et al. (2008) Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril 90: 737–43. [DOI] [PubMed] [Google Scholar]
- 34. Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, et al. (2005) Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG 112: 1384–90. [DOI] [PubMed] [Google Scholar]
- 35. Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z (2006) Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril 85: 592–6. [DOI] [PubMed] [Google Scholar]
- 36. Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, et al. (1989) Mullerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology 125: 1060–6. [DOI] [PubMed] [Google Scholar]
- 37. Hirobe S, He WW, Gustafson ML, MacLaughlin DT, Donahoe PK (1994) Müllerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Biol Reprod 50: 1238–43. [DOI] [PubMed] [Google Scholar]
- 38. Rey R, Sabourin JC, Venara M, Long WQ, Jaubert F, et al. (2000) Anti-Müllerian hormone is a specific marker of sertoli- and granulosa-cell origin in gonadal tumors. Hum Pathol 31: 1202–8. [DOI] [PubMed] [Google Scholar]
- 39. Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, et al. (1995) Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136: 4951–62. [DOI] [PubMed] [Google Scholar]
- 40. Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, et al. (2005) Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod 20: 3178–83. [DOI] [PubMed] [Google Scholar]
- 41. Tremellen KP, Kolo M, Gilmore A, Lekamge DN (2005) Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol 45: 20–4. [DOI] [PubMed] [Google Scholar]
- 42. La Marca A, Stabile G, Artenisio AC, Volpe A (2006) Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 21: 3103–7. [DOI] [PubMed] [Google Scholar]
- 43. Takahashi M, Hayashi M, Manganaro TF, Donahoe PK (1986) The ontogeny of mullerian inhibiting substance in granulosa cells of the bovine ovarian follicle. Biol Reprod 35: 447–53. [DOI] [PubMed] [Google Scholar]
- 44. Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, et al. (2002) Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143: 1076–84. [DOI] [PubMed] [Google Scholar]
- 45. La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, et al. (2004) Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod 19: 2738–41. [DOI] [PubMed] [Google Scholar]
- 46. Lukas-Croisier C, Lasala C, Nicaud J, Bedecarrás P, Kumar TR, et al. (2003) Follicle-stimulating hormone increases testicular Anti-Mullerian hormone (AMH) production through sertoli cell proliferation and a nonclassical cyclic adenosine 5′-monophosphate-mediated activation of the AMH Gene. Mol Endocrinol 17: 550–61. [DOI] [PubMed] [Google Scholar]