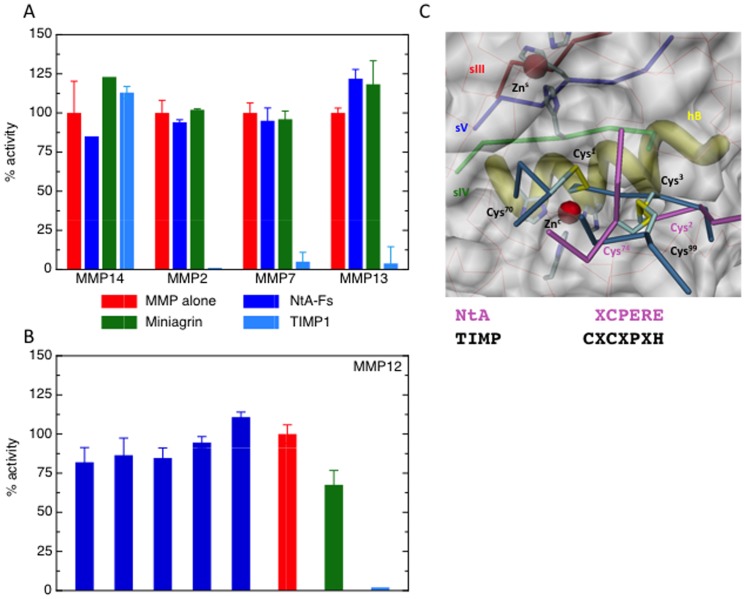
Figure 1. Agrin does not inhibit MMPs.
Comparison of inhibitory action of miniagrin, NtA-Fs and TIMP-1 for: (A) MMP-2 (1 nM), MMP-7 (5.6 nM) and MMP-14 (4 nM) and MMP-13 (10 nM) with 50 nM TIMP-1, 100 nM Nta-FS and 1 µM miniagrin; (B) MMP-12 (1 nM) with a range of NtA-FS concentrations, 1 µM miniagrin and 50 nM TIMP-1; Values are expressed as a percentage of the uninhibited MMP activity +/− standard deviation. (C) Superimposition of NtA (Cα-backbone in pink) and TIMP-1 (Cα-backbone in steelblue) projected into the active site cleft of MMP-3 (pdb-code 1uea). Essential elements of MMP are highlighted in different color schemes (sIII-v, hB and both histidine fingers). The key disulfide bridges of TIMP-1 (Cys1–Cys70 and Cys3–Cys99) and NtA (Cys2–74) are labeled accordingly. Structural (Zns) and catalytic zinc (Znc) ions are shown as red spheres. The N-terminal sequences for NtA (in pink) and TIMP-1 (in black) reveal the missing Cys in position 1.