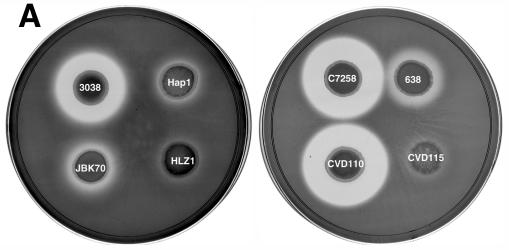
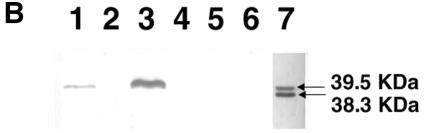
FIG. 2.
(A) Protease activity of V. cholerae hap mutants and their isogenic parent strains on milk-containing medium. Bacteria were grown for 16 h at 37°C, pelleted by centrifugation, and 10 μl of the supernatant was spotted onto the plates and incubated for 48 h at 37°C. (B) Western blot analysis of Hap production. Overnight bacterial cultures were subcultured (at a dilution of 1:1,000) into 5 ml of TSB medium, and incubated at 30°C with shaking (200 rpm) for 20 h. The cultures were centrifuged, filtered, and concentrated through Centricon-30 centrifugal filters. The retentate was adjusted to 0.05 ml, 50-μl aliquots were subjected to SDS-12% PAGE, and the proteins were transferred to a polyvinylidene difluoride membrane. Hap was detected by using a rabbit anti-Hap serum and peroxidase-conjugated goat anti-rabbit immunoglobulin G. Lane 1, C7258; lane 2, 638; lane 3, 3038; lane 4, Hap1; lane 5, JBK70; lane 6, HLZ1; lane 7, pure Hap (2.5 μg). Molecular masses were calculated with reference to SDS molecular mass standards from Bio-Rad laboratories.