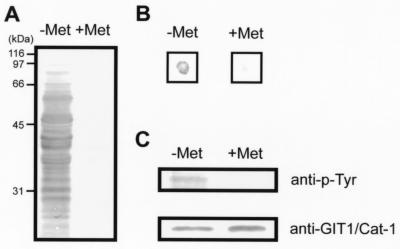
Figure 2.
Substrate identification with the yeast substrate-trapping system. (A) Yeast proteins were highly tyrosine-phosphorylated only when v-src was induced in the absence of methionine (−Met), whereas almost no tyrosine-phosphorylation was observed in the presence of methionine (+Met). Yeast lysates (70 μg) were analyzed by Western blotting with antiphosphotyrosine antibody. (B) β-Galactosidase filter-lift assay for clone 10–1. A filter placed on the plate lacking leucine, tryptophan, and methionine (Left) and lacking leucine and tryptophan (Right) was streaked directly with clone 10–1 and pBridgeLexA/ζICR-D1902A/v-src cotransformants, incubated at 30°C for 2 days, and developed for 3 h for color detection. (C) The prey consisting of the GAL4 activation domain fused to GIT1/Cat-1 was actually tyrosine-phosphorylated by v-src induction (−Met). Yeast cell lysates (750 μg) were immunoprecipitated with anti-GIT1/Cat-1 antiserum and analyzed by Western blotting with antiphosphotyrosine antibody (Upper) and anti-GIT1/Cat-1 antiserum (Lower).