Abstract
Measurement of resting blood flow to the skin and other organs is an important indicator of health and disease and a way to assess the reaction to various stimuli and pharmaceutical interventions. However, unlike plasma ions such as sodium or potassium, it is difficult to determine what the proper value for resting blood flow really is. Part of the problem is in the measurement of blood flow; various techniques yield very different measures of skin blood flow even in the same area. Even if there were common techniques, resting blood flow to tissue, such as the skin, is determined by the interaction of a plurality of factors, including the sympathetic nervous system, temperature, pressure, shear forces on blood vessels, tissue osmolality, and a variety of other stimuli. Compounding this variability, the blood flow response to any stressor is reduced by free radicals in the blood and diminished by aging and diabetes. Race also has an effect on resting blood flow to the skin. All these factors interact to make the exact resting blood flow difficult to determine in any one individual and at any one time. This review examines the main techniques to assess blood flow, the factors that alter blood flow in the skin, and how aging and diabetes affect blood flow. Recommendations for the measurement of resting blood flow are presented.
Keywords: aging, circulation, diabetes, free radicals
Introduction
Endothelial dysfunction is a common denominator in many pathologies in man. It is associated with aging, diabetes, high blood triglycerides, cigarette smoking, and other contributors to inflammation.1–5 With so many factors that can alter blood flow in the skin and other organs, the concept of “what is” normal blood flow is important to know as a basis of comparison to the diseased state. Numerous factors can influence resting blood flow. Skin blood flow in hairy skin, for example, is controlled through the release of compounds synthesized in vascular endothelial cells, some of which reduce circulation and some of which increase circulation through constricting or relaxing vascular smooth muscle.6,7 Thus the vascular endothelial cell controls the contractile state of vascular smooth muscle.8 This article will encompass a brief review of the methods used to measure skin blood flow, the endothelial cell, and major factors that alter skin blood flow.
Methods Used to Measure Skin Blood Flow
Venous Occlusion Plethysmography
Plethysmography has been used since the 1900s to measure limb blood flow. It does not measure skin blood flow alone, but measures blood flow to the entire limb. The oldest technique involves placing the arm or leg in a water bath and measuring the displacement of water when a venous occlusion cuff is placed on the thigh or axilla.5 Because veins are capacitance vessels, when a pressure of 50 mm Hg is applied to a venous occlusion cuff, the veins fill and the volume of the arm changes.9 This displaces water, the amount of which is equal to the incoming blood flow in the limb. This technique requires careful sealing of the limb in the bath through windows which usually leak constantly. This technique will only work on limbs. Water volume plethysmography was replaced by the use of a Silastic tube that encircled the limb and was filled with mercury.5,10–12 Because the limb increases volume when the veins are occluded, this would, in turn, stretch the Silastic tubing and change the electrical resistance in the mercury. This technique has been used continuously since the 1950s as a gold standard for measuring limb blood flow.13,14 There are, however, limitations. First, skin and muscle blood flows are measured together, and there is no way to partial out skin blood flow. Second, unless the Silastic is kept stretched by a small weight, it responds nonlinearly to stretch and has a hysteresis in stretching and relaxing that causes an error in blood flow recordings. In addition, for high blood flows, the venous cuff must also be inflated in less than 0.5 s or the veins will fill before the cuff fully inflates. The hand circulation must also be eliminated by an arterial occlusion cuff since it is so high that it will fill the veins in the arm very quickly. Finally, since the actual strain gauge is only on a fraction of the arm, it may give false low recordings. This has been seen when an air cuff is placed over the whole arm to measure volume changes.15 For high flow rates, the Dohn air cuff gave higher flow reading than the mercury in rubber strain gauge systems.15 This technique is also very motion sensitive. This technique provides actual blood flow in cc/100 g tissue per minute.
Ultrasound
Ultrasound has been used for many years to measure blood flow. In large and small arteries, ultrasound can be used to measure the blood flow velocity by the Doppler technique, and when multiplied by vessel diameter, it can provide the blood flow.16–18 This technique works for large and small arteries but does not give tissue blood flows.19 In a pulsed ultrasound system, a time delay is placed between the transmission of a pulse and the recording. The returning signal generates a difference from the transmitted frequency due out the velocity of the blood cells under the sampling area.20 The principal defect is that the angle of the ultrasound head must always be perpendicular to the artery, and the larger the artery, the better. Equipment frequency and resolution is also important.19 Movement of the patient induces a large error in the recording.21 Linear pulse array ultrasound heads work best in the 5–10 MHz range.21 A major problem is plaque, which can occlude the signal and cause false low blood flow recordings.21 Ultrasound cannot provide microvascular blood flows.
Laser Doppler Blood Flow
Laser Doppler techniques are the most common techniques for measuring skin blood flow.21,22 Like ultrasound, it depends on the change in frequency between an applied laser beam and the returning beam of light to determine the velocity of blood flow. The transmission of light is used to assess red cell concentration, which, when multiplied by the velocity, provides blood flow.23 The unit of blood flow is the “flux.” The laser used on the skin typically has a diameter of approximately 1 mm.24 If the dot is in a fixed location, it is called a laser Doppler flow meter. If the beam scans the skin, a large skin area can be scanned and a laser Doppler image of the skin surface reflecting blood flow can be seen.25–27 These devices are called laser Doppler imagers. Another technique is laser speckle flow imagers. They project a constant speckle laser pattern on the skin to obtain rapid pictures of flow, typically 25 per second compared with 2 min using a laser Doppler imager.28 The speed is a sacrifice for depth of penetration, which is less than 1/2 dermal thickness. Depending on laser frequency and power, all techniques have different areas they cover and different penetration into tissue.
There are numerous pros and cons to this technique. First, skin blood flow varies continuously because of vasomotor rhythm and respiration. Blood flow increases slightly during exhaling and is reduced slightly during inhalation. If flow is sampled too quickly, it may be high or low, depending on respiration. Blood flow also varies with the beat of the heart. During systole, it increases, and it decreases during diastole. Thus one important variable is recording for at least 20 s to get an average blood flow in a given region of the skin.
Skin light scattering properties as well as that of other tissues alters the depth of penetration of laser Doppler measurements of blood flow.29 The smallest depth of penetration is with single fiber optic fiber flow meters, where depth can be as little as 146 mm. Further, for single-fiber probes, a freckle or even an ink spot on the skin can occlude the transmission of light and show small or negligent blood flows. If the probe is placed near the blood supply of a hair follicle or sweat gland, the reading can be very high. Single fiber probes can also be subject to motion artifacts. Even the slightest movement can alter the apparent blood flow. The frequency of light is also important in determining depth of penetration. Red laser light penetrates about 2/3 of dermal thickness, while infrared penetrates full dermal thickness in the skin.30
Therefore, the most accurate systems are the large beam laser Doppler imagers, because these scan slowly over a large area and, with an infrared beam, have good penetra-tion. But these are also motion sensitive, and therefore, the subject must sit or lie comfortably.31 Acclimatization should be 20–30 min as blood flow is often not stable when a person walks because exercise increases skin circulation as does high environmental temperatures.31 Ambient light levels should be kept low because they can interfere with flow recordings. Pressure should not be applied to the skin, as this will change blood flow.32 Talking and moving should be avoided during imaging because these will alter the skin blood flow.
Given these limitations, this is presently the best technique to measure blood flow in the skin.
Thermostrom
One novel technique for measuring skin blood flow is by using a heated thermistor pair.33 This technique uses a pair of matched temperature-sensing devices called thermistors. These devices change electrical resistance with temperature. By placing a single coil of wire around one thermistor, a current through the coil is used to heat one thermistor 1 °C above the other. If the device is placed on the skin, the greater the skin blood flow, the greater will be the current needed to heat the one thermistor. Therefore, current is proportional to blood flow. The advantage of this device is that it samples over a large area (typically 0.5 cm2) and responds slowly so that motion artifacts are at a minimum. The depth of penetration is not clear and depends on the passive thermal properties of the skin. Blood flow is in relative units.
Hertzman Photoelectric Plethysmography
Light (infrared) is absorbed by blood going through the skin. In the 1940s, Hertzman discovered this, and by placing an infrared light source at 45° to the skin and a photocell at 90° to the light source, the transmission of infrared light provides a measure of tissue blood flow.34–37 One problem is motion. Any movement causes such a large artifact that flows cannot be recorded. They also only give relative blood flows and not absolute blood flows.38 However, this technique is cheap and is still used today for many applications.39,40 It can be used for skin or even for organs with implantable probes.41
Impedance
Tissue impedance gives a measure of blood flow.42 It does not measure skin blood flow independent of limb blood flow. This technique requires four electrodes (two on the upper and two on the lower limb). It uses a change in tissue conductivity to measure blood flow.43,44 This technique is very subject to body hydration levels and to race and age differences in tissue conductivity. Limb fat content also creates an error.
Radioactive Isotopes
Radioactive isotopes have been used to measure skin blood flow. For example, Xenon-133 clearance has been used in many studies.45,46 Radioactive iodine and krypton have also been used as well as phosphorous32.47 Radioactive microspheres have also been used and can be labeled with nuclides C41, Ce51, Cr,85Sr,95 Nb, or 46SC.48 The common problem with these techniques is that they are expensive, and for some of the ions like xenon, they are absorbed by body fat, which changes the clearance rate and creates an error.49
Therefore, to understand skin blood flow, it is best to start with the endothelial cell in the vascular system.
The Vascular Endothelial Cell
The start of any discussion of what resting blood flow is begins with the lining of the blood vessels, the vascular endothelial cell. The endothelial cell is the final interphase between the blood and the surrounding vascular smooth muscle.7,8 The contractile state of vascular smooth muscle is controlled by factors released by these endothelial cells.50 Two classes of compounds are released, broadly called vascular relaxation factors and vasoconstrictors. They diffuse through the endothelial cell, into vascular smooth muscle, and into the circulation.51,52 Vascular relaxation factors are fat-soluble chemicals that increase potassium permeability in the surrounding vascular smooth muscle and impair calcium permeability.53 Since the membrane potential of an excitable cell is largely due to the potassium equilibrium potential, endothelial cells and smooth muscle cells are both electronegative on the inside with respect to the outside.3 However, in smooth muscle, there is also some sodium permeability in the resting cell membrane. Sodium, a positively charged ion found in the blood and largely absent intracellularly in vascular smooth muscle, readily diffuses down its concentration gradient from blood into muscle, carrying positive charges into muscle.7 These charges reduce the electronegativity of the intracellular plasma membrane. Therefore, an increase in potassium permeability will neutralize some of these positive charges, making the membrane potential more negative and moving it farther away from threshold.54–56 Hyperpolarization of the smooth muscle membrane and reduced calcium permeability relax vascular smooth muscle and allow an increase in skin blood flow.
Compounds released by vascular endothelial cells, called vasoconstrictors, are also fat-soluble compounds and diffuse into blood and vascular smooth muscle.6,57 In vascular smooth muscle, they bind to calcium channels, increasing calcium permeability. Thus, for a given action potential, more calcium enters and smooth muscle constricts, thereby limiting circulation by reducing the lumen size of the arteriole.57
At any one time, both factors are being released simulta-neously. When the balance of release tilts more in one direction than in the other, the luminal size of the arteriole changes and hence, circulation increases or decreases, respectively.58–61
A principal vasodilator is nitric oxide.62 It is released by vascular endothelial cells and synthesized by the enzyme endothelial nitric oxide synthetase.63 Nitric oxide is derived by the bioconversion of l-arginine to l-citrulline, two common amino acids. L-arginine has three nitrogens in its structure.2,62,64 The enzyme nitric oxide synthetase removes one of the nitrogens and oxygens, forming another amino acid, L-citriline,58,65 leaving nitric oxide as the byproduct of the reaction. Nitric oxide diffuses from the endothelial cell into vascular smooth muscle, where it binds as a ligand to the enzyme guanalyl cyclase.66 This activates the enzyme, allowing it to form cyclic guanosine monophosphate, the chemical mediator that increases potassium permeability and reduces calcium permeability in smooth muscle.67 This, in itself, hyperpolarizes smooth muscle, making it harder to excite and promoting relaxation. To hyperpolarize smooth muscle cells further, there are gap junctions connecting endothelial cells to smooth muscle that allow hyperpolarization to occur on smooth muscle when endothelial cells hyperpolarize due to the presence of substances that cause the release of nitric oxide.68
Another class of compounds that is associated with vascular control is the prostaglandins. Prostaglandins are derived from the fatty acid arachidonic acid, commonly found throughout the body. Arachidonic acid is in high concentrations in cell membranes.7 In the membrane of the endothelial cell and other cells in the body is an enzyme called cyclooxygenase.69 Cyclooxygenase adds oxygen to couple two of the carbons in arachidonic acid, forming the first member of a class of compounds called prostaglandins. Some of the prostaglandins cause vasodilation, while others cause vasoconstriction. For example, one member of the class, called prostacyclin, is a prostaglandin-mediated vasodilator compound.69 Prostaglandin H2, on the other hand, causes increased sodium permeability in vascular smooth muscle via cyclic adenosine monophosphate and is a potent vasoconstrictor.
The cell membrane of the vascular endothelial cell contains receptors. The insulin receptor, for example, causes the activation of the phosphatidylinositol 3'-kinase pathway when insulin binds to the receptor.70 This pathway is activated by several receptors, including G-protein-coupled receptors (e.g., chemokine receptors), estrogen, tyrosine kinases (e.g., vascular endothelial growth factor receptors), integrins, and death receptors (e.g., tumor necrosis factor-a receptor).71–73 In turn, phosphatidylinositol 3-kinase signaling promotes nitric oxide release (through endothelial nitric oxide synthase phosphorylation), angiogenesis (through Ras homolog A), endothelial progenitor cell recruitment, and cell viability.74
Factors that Alter Tissue Blood Flow
Tissue Local Pressure and Occlusion
When pressure is applied to the skin, affected tissues can become hypoxic and increase the concentration of metabolites that dilate arterioles and decrease vascular resistance in healthy skin.75,76 When pressure is applied to skin, skin blood flow increases to prevent damage to tissue. However, with ever-increasing pressure, the tissue hydrostatic pressure rises above arterial pressure, and skin blood flow is eventually occluded.65,76 Once the pressure is released, local blood flow is temporarily elevated due to increased vascular metabolites such as hydrogen ions.65,76 The temporary increase in blood flow is called a reactive hyperemia and serves to reoxygenate tissue and flush vasodilator metabolites from the tissue.55 The size of the reactive hyperemia is proportional to the duration and extent of vascular occlusion during the time that pressure is applied. However, this response is blunted in older people and especially in people with diabetes as shown in Figure 1.
Figure 1.
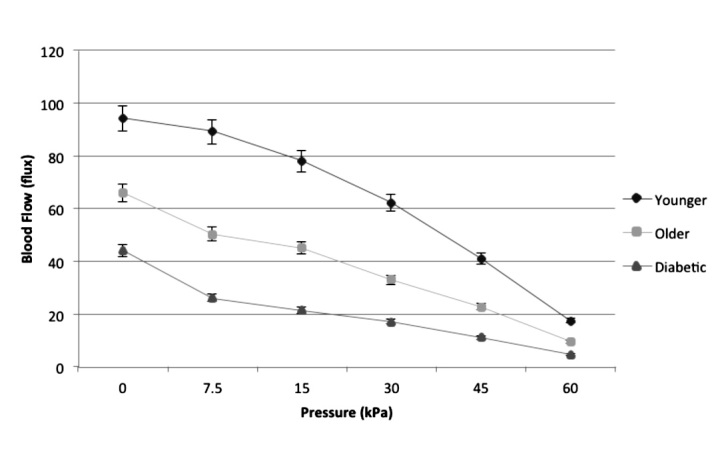
Vascular occlusion on the foot due to application of 30 s of pressure in three groups of subjects in 24 °C global temperature with standard deviation bars. Data were collected on 15 older subjects, 15 subjects with diabetes, and 15 younger subjects. Blood flow is measured in the skin by a laser Doppler flow meter. Reproduced with permission from Diabetes Technology & Therapeutics.76
Full occlusion of circulation creates a much larger reactive hyperemia after the occlusion is removed. A standard test of endothelial function is to occlude circulation for 4 min and then measure the reactive hyperemia that follows.77 For young individuals, as shown in Figure 2, the hyperemia lasts less than 2 min in the skin. It initially increases approximately 20-fold and then returns to a normal circulation after 2 min. The total increase in blood flow over the 2 min period is called excess blood flow and represents the repayment of metabolic debt in the tissue incurred during occlusion.
Figure 2.
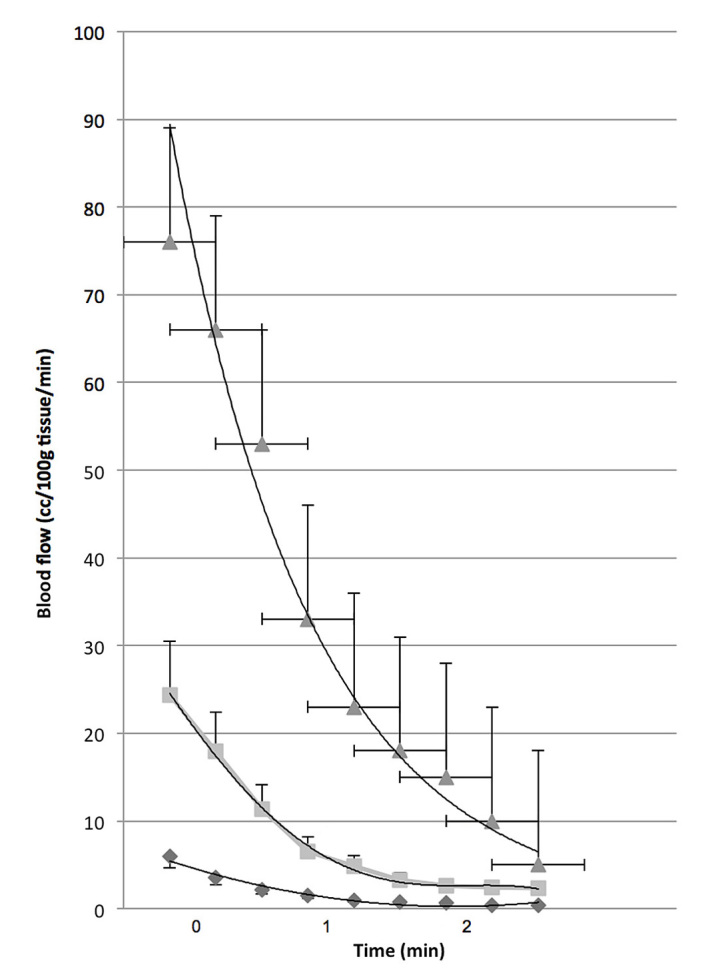
The blood flow of the arm from the elbow to the wrist assessed by venous occlusion plethysmography for 2 min after 4 min of vascular occlusion in young (triangle), older (squares), and people with diabetes (diamond). Thirty controls and 16 subjects with type 2 diabetes participated in this series of experiments to examine the interrelationships between age, diabetes, and endothelial cell function. The mean age of subjects with diabetes was 61.2 ± 10.1 years and for the nondiabetic group 55.3 ± 9.7 years. Reproduced with permission from BMC Endocrine Disorders.78
Effect of Temperature on Skin Blood Flow
Pennes first established that circulation can protect the skin from damage that might occur after application of a heat source.79,80 The increase in skin circulation in response to a sustained heat locally applied to the skin involves two separate mechanisms: a quick-acting response and a slow-acting response. For a rapidly applied heat source, skin circulation does not respond fast enough to dissipate heat, and only the passive properties of skin can protect skin from damage.81,82 When a sustained heat load is applied to skin, in the first few minutes, tactile sensors in skin cause a progressive vasodilatation in skin blood vessels mediated by substance P and other vasoactive neuropeptides such as calcitonin gene-related peptide released from sensory nerves.83–85 As this initial response subsides, there is a more prolonged increase in skin blood flow mediated by nitric oxide released from vascular endothelial cells. While the initial increase in skin blood flow is mediated by TRPV1 voltage-gated calcium channels on tactile sensory nerves, the sustained blood flow response to heat is mediated by the enzyme endothelial nitric oxide synthetase activated by calcium released intracellularly through TRPV4 voltage-gated calcium channels.54,55,64 Skin circulation remains elevated to remove heat from skin while heat is applied.52,56,79,86 While several vasodilators respond to heat in younger individuals, with aging, nitric oxide is the predominant pathway associated with sustaining the heat-mediated vasodilatation.86–88 The response to heat is illustrated in Figure 3 for younger subjects, older subjects, and subjects with diabetes (discussed later).
Figure 3.
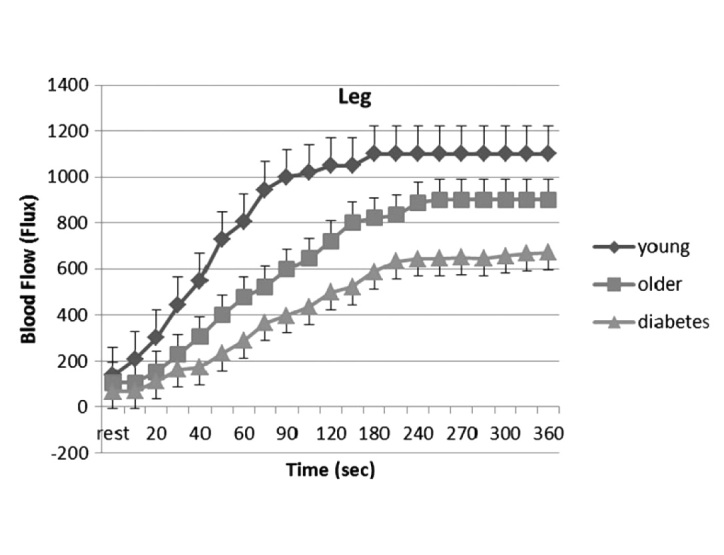
Average blood flow response of the 30 subjects (± the appropriate SD) during heating by a thermode for 6 min for the leg: younger group (diamond), older group (square), and subjects with diabetes (triangle). Blood flow is measured by laser Doppler imager of the skin. The mean ages of the 10 subjects in each group was younger, 25.1 ± 2.2 years; older, 59.1 ± 6.1 years; and subjects with diabetes, 61.3 ± 7.9 years. The average duration of diabetes was 2–13 years, and the average hemoglobin A1c was 7.4 ± 1.3%. Reproduced with permission from Diabetes Technology & Therapeutics.89
Moist Heat and Skin Blood Flow
While the concept of heat increasing circulation has been known for decades, the interaction of other stimuli with heat has not been well studied. For local heat, for example, if electrical stimulation is applied to skin at currents less than 20 mA when the skin is warmed at the same time, the blood flow response of the skin will be greater than that of the heating alone.83,90 Conversely, if the skin is cooled, blood flow will be reduced in proportion to the skin temperature. When the skin is cooled to 30 °C, the same electrical stimulation has no effect on skin blood flow.91 This phenomenon is explained best by the fact that multiple stimuli operate through the same voltage-gated channels on vascular endothelial cells. A type of voltage-gated calcium channel, the TRPV4 channel, is common to transduction of vertical pressure, shear pressure, sensation for warmth, response to acetylcholine and blood and tissue osmolarity.65 By sharing the same receptor, these stimuli interact with each other. If the cell osmolarity is high, then the blood flow response to heat is high, while low cell osmolarity reduces the blood flow response to heat92 as shown in Figure 4. Figure 4 shows the blood flow response to 20 min of sustained heat to bring the skin to three different skin temperatures, 38, 40, and 42 °C. The graph is the average of 10 subjects, showing the blood flow response to a dry heat source (ThermaCare heat wraps) and a chemical moist heat wrap, where heating is by water vapor at 100% humidity.
Figure 4.
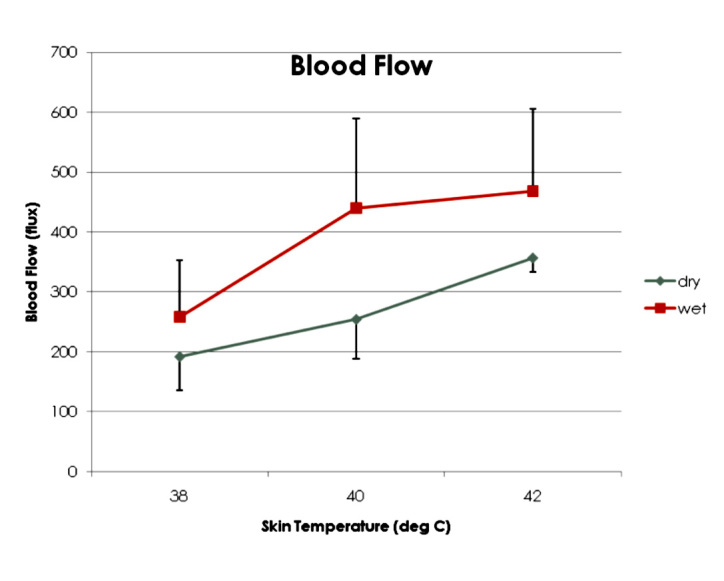
The blood flow response of the skin to heat at three temperatures with a dry and moist heat source. Blood flow is measured by laser Doppler imager of the skin. Data represent 20 young subjects, average age 24.1 ± 2.4 years. Reproduced with permission from Journal of Medical Engineering & Technology.91
Aging and Diabetes
Both aging and diabetes alter skin blood flow. Aging reduces skin blood flow.78 Part of the mechanism is due to endo-thelial cell damage. This damage reduces the release of vasodilators such as prostacyclin and impairs nitric oxide release or bioactivity.93 In some studies, it has been demonstrated that nitric oxide production is impaired due to a defect in the enzyme that produces nitric oxide, nitric oxide synthetase.93 Other studies point to reduced availability of the amino acid l-arginine that is the precursor for the production of nitric oxide.94 Many studies point to reduced bioavailability of nitric oxide due to oxidation by free radicals to peroxynitrite.77 Peroxynitrite has no biological activity as a vasodilator, and therefore the balance in the endothelial cell moves toward vaso-constriction of the arteries and blood flow is reduced at rest and in response to stress.57 For example, when occlusion is applied to the arm as was seen in Figure 2, in older people, as aging progresses, there is a reduction in blood flow at rest and in response to occlusion. This is further exacerbated in people with diabetes. Here, aging and diabetes both cause a reduction in skin blood flow at rest and in response to occlusion. This is best shown in Figure 5. Illustrated here is the total excess blood flow after occlusion in young, old, and people with diabetes over the 2 min postocclusion period as shown in Figure 2. The total excess blood flow is the total blood flow above rest in the 2 min period after vascular occlusion for 4 min. As shown in this figure, the older the person, the less the total increase in blood flow after occlusion. Interestingly, the two lines showing a reduction in excess blood flow with increasing age for subjects with and without diabetes are parallel with identical slopes; the only difference is that diabetes reduces blood flow in response to occlusion at any age. The response to heat on the skin is similar; it is reduced by both aging and diabetes.
Figure 5.
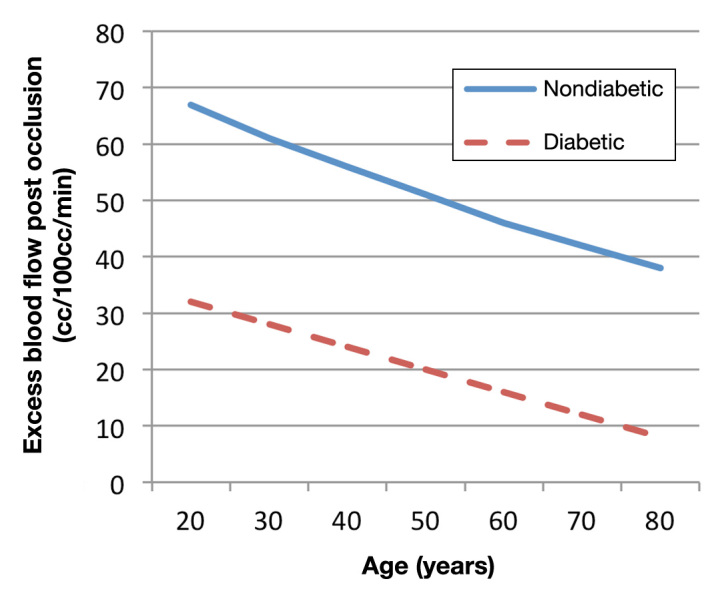
Excess in blood flow over rest during 2 min after vascular occlusion in younger and older people with and without type 2 diabetes. The regression equation for the subjects with diabetes is y = -0.3945x + 39.269 and for the older age-matched subjects is y = -0.5092x+ 77.707. There was no statistical difference between the slopes. Data are represented on 22 subjects with diabetes and 26 age-matched controls. Blood flow is measured on the arm by mercury in rubber plethysmography. Data are from Figure 2, calculated by integration as described in the text. Reproduced with permission from BMC Endocrine Disorders.78
Vitamins and Blood Flow
Vascular endothelial function is critical for the health of the organs in the body.95 It was once believed that endothelial dysfunction was only seen with age and diseases such as diabetes.57 This reduction in endothelial function causes an impaired blood flow response to stressors on the skin such as heat and pressure and reduced blood flow to vital organs such as the heart and kidney, causing senescence in the cardiovascular system.7 With what the World Health Organization calls an epidemic of obesity and diabetes, endothelial impairment is being seen even in young people.95
One factor believed to be a principal cause of endothelial dysfunction is high concentrations of free radicals in the body. Free radicals are commonly produced and neutralized in the body.96 Some free radicals are produced and used for cellular communication, while others are produced as a natural product of cellular metabolism.26,97–99 For example, nitric oxide, a commonly produced free radical, is released from mitochondria and vascular endothelial cells to increase circulation in the tissue.26
Two to five percent of oxygen used by mitochondria forms free radicals.100 With exercise, oxidative phospho-rylation increases dramatically, increasing the production of free radicals.101 For example, using electron spin resonance spectroscopy,102 there was a 70% increase in free radical production from electrically stimulated rat muscle compared with controls. It is of no surprise, then, that exercise is considered an inflammatory process.101
While nitric oxide is a free radical, it is relatively weak compared with other free radicals such as hydrogen peroxide. When such superoxides react with nitric oxide, they bio convert it to an inactive form such as peroxynitrite (ONOO), a free radical with no influence on circulation.95 Bioconversion of nitric oxide to peroxynitrite is believed to be one of the mechanisms associated with the reduction in circulation at rest and during stress in older people and people with diabetes.95
A common measure of endothelial function is flow-mediated vasodilation (FMD), which is mediated by shear stress on large arteries through a prostaglandin mechanism95 and is a measure of macrovascular function. High free radical levels in young men have been shown to be negatively correlated with FMD (of arteries).59,103 Another measure of endothelial function is the skin response to local heat.95 Numerous studies have examined the administration of vitamins A, C, and E, known potent antioxidants, on free radicals and performance. For example, since free radicals are associated with muscle soreness and the inflammatory response to exercise, various studies have shown that oral doses of vitamin E or C increase the antioxidant capacity and a reduction in muscle soreness.104,105 In a study of chronic smokers, FMD was reduced in smokers but increased in the same population with administration of vitamin C (1000 mg) and E (500 U) for 25 days. Another study of smokers also showed a low FMD, which was reversed with administration of 600 IU of vitamin E for 4 weeks.106 Gross measures of vascular function such as protection from damage from myocardial infarction have been shown with as little as 14 days of vitamin E supplementation by scavenging free radicals, improving antioxidants, and maintaining Ca(2+) levels.107,108
Considering the fact that numerous studies have examined the benefit of vitamins in reducing free radicals in the body,109–111 it is surprising that very little has been done to see if vitamin supplementation can alter direct measures of endothelial function, such as the response to local heat or occlusion-mediated vasodilatation. However, as shown in Figure 6, when young subjects took high doses of vitamin C, E, and coenzyme Q10 for 2 weeks, there was a greater blood flow response at rest and in response to heat.
Figure 6.
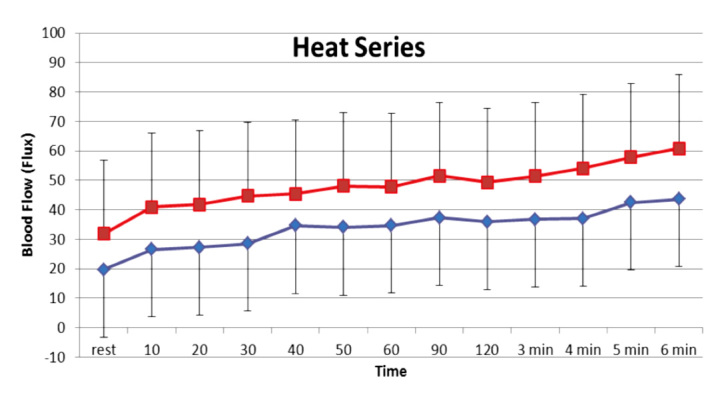
Blood flow response of the skin to heat over 6 min in two age-matched groups of young subjects; one group (squares) took vitamins for two weeks prior to measurements. Data are on 18 control subjects and 18 who took vitamins for two weeks. Blood flow is measured on the skin by a laser Doppler flow meter. Reproduced with permission from Anatomy & Physiology.112
Race
Blood flow in skin and other tissues is controlled by vascular endothelial cells. Studies show that different racial populations have different genes that can alter endothelial function.113 For example, people from Thailand and other Northern and Southern Asian countries have a “thrifty gene.”113,114 This gene was developed to protect this population from starvation and alters a nuclear transmitter PPAR.114 The gene was developed to allow people from Asia to exist on a carbohydrate diet, low in fat. The thrifty genotype involves many single nucleotide polymorphisms.115 When this population eats a high-fat diet, free radicals are produced in endothelial cells, which impairs their function.77 Figure 7 shows the blood flow response to heat in Koreans versus Caucasians that were age matched. Skin was heated over 6 min to a temperature of 42 °C. During heating, there was no difference in the tactile sensory phase (first 2 min) but a significant difference in the nitric-oxide-mediated phase in response to heat. In these same groups of subjects, Koreans also had a diminished blood flow response to occlusion for 4 min on the arm (Figure 8).
Figure 7.
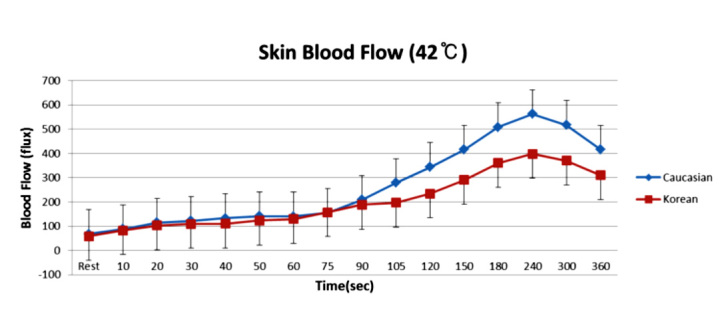
Mean ± standard deviation of blood flow (flux) measured during the exposure to heat at 42 °C in 10 Caucasians and 10 Koreans at rest and over a period of 360 s. Blood flow is measured by a laser Doppler flow meter. Reproduced with permission from Medical Science Monitor.116
Figure 8.

Mean ± standard deviation of blood flow (flux) measured during the 4 min period of occlusion and the 2 min period following the release of the occlusion cuff in 10 Caucasians and 10 Koreans. Blood flow was measured by a laser Doppler imager of the skin. Reproduced with permission from Medical Science Monitor.116
High-fat foods are especially hard on Koreans due to the “thrift” genes. If age-matched Koreans and Caucasians (10 in each group) are given a low- and high-fat meal, the oxidative stress 2 h after the meal, as shown by malondialdehyde in the blood in Figure 9, is much higher than the Asians. But if both groups are placed on a vitamin regime high in vitamin C and E and Q10, potent antioxidants, for 2 weeks and the meals are repeated, the oxidative stress is reduced in the Koreans. Similarly, after ingesting a high-fat meal, Koreans have a diminished blood flow response to occlusion as shown in Figure 10. After 2 weeks of vitamins, Figure 11, the blood flow response to occlusion is not altered by a high-fat meal in these same Koreans.
Figure 9.
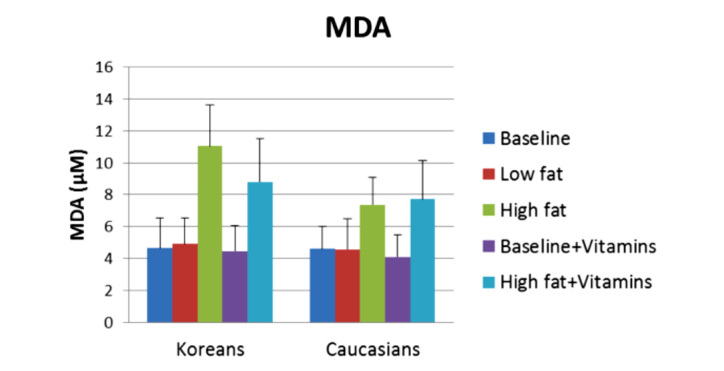
Malondialdehyde is a measure of blood-born free radicals used to assess oxidative stress in the body. Illustrated here is the average malondialdehyde in venous blood in 10 Caucasians and 10 Koreans at baseline and 2 h after a high-fat and low-fat meal both before and after a 2-week vitamin regime. MDA, malondialdehyde.
Figure 10.
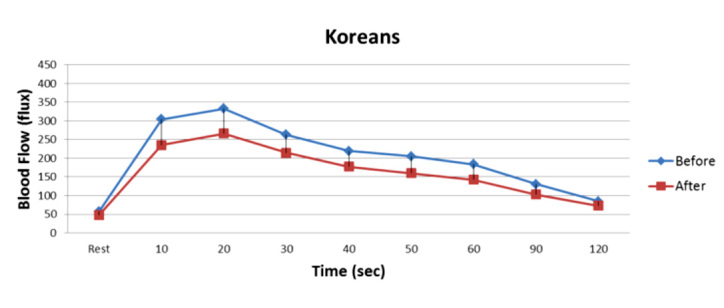
Illustrated here is the blood flow response to 4 min of occlusion in 10 Koreans 2 h after a high-fat meal before vitamin administration. Blood flow was measured in the skin by a laser Doppler imager. Blood flow postocclusion was significantly lower after a high-fat meal (2 h post; analysis of variance p < .05).
Figure 11.
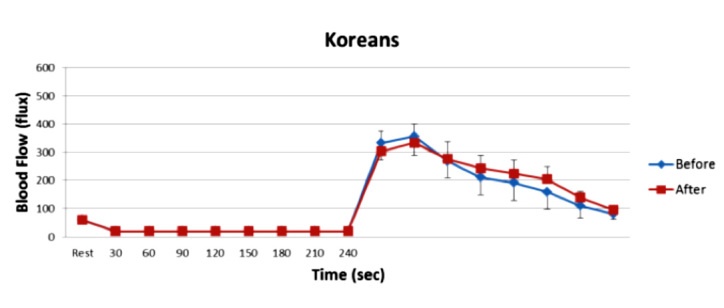
Illustrated here is the blood flow response to 4 min of occlusion in 10 Koreans after a high-fat meal before and after two weeks of vitamin administration. Blood flow was measured in the skin by a laser Doppler imager. There was no significant difference in the blood flow response to occlusion before and after the high-fat meals (analysis of variance p > .05).
Summary
Resting blood flow is a balance between multiple factors that cause vasodilation and constriction. Even at similar skin temperatures, other factors such as skin moisture, pressure, age, diabetes, and exposure to free radicals alter the skin blood flow. Therefore, skin blood flow at rest and in response to stress is a balance of many factors, and no one number represents this value. Thus resting blood flow is just too dependent on too many factors to be represented by a single value. However, certain conditions make studies more reliable if skin blood flow is to be measured. First, the most reliable technique to measure blood flow is a laser imager where at least an area of 1 × 1 cm2 is measured with a slow scan over a 1 min period. Next, subjects should rest at least 30 min before measurements in a thermally neutral room. 140where there is little movement. Room light should be dim, and infrared light should be avoided. Subjects should be tested at the same time of the day, and high-fat meals should be avoided within 4 h of testing.
Glossary
Abbreviations
- (FMD)
flow-mediated vasodilation
References
- 1.Petrofsky J, Berk L, Alshammari F, Lee H, Hamdan A, Yim JE, Patel D, Kodawala Y, Shetye G, Chen WT, Moniz H, Pathak K, Somanaboina K, Desai R, Dave B, Malthane S, Alshaharani M, Neupane S, Shenoy S, Nevgi B, Cho S, Al-Nakhli H. The effect of moist air on skin blood flow and temperature in subjects with and without diabetes. Diabetes Technol Ther. 2012;14(2):105–116. doi: 10.1089/dia.2011.0128. [DOI] [PubMed] [Google Scholar]
- 2.Petrofsky J, Alshahmmari F, Yim JE, Hamdan A, Lee H, Neupane S, Shetye G, Moniz H, Chen WT, Cho S, Pathak K, Malthane S, Shenoy S, Somanaboina K, Alshaharani M, Nevgi B, Dave B, Desai R. The interrealtionship between locally applied heat, ageing and skin blood flow on heat transfer into and from the skin. J Med Eng Technol. 2011;35(5):262–274. doi: 10.3109/03091902.2011.580039. [DOI] [PubMed] [Google Scholar]
- 3.Petrofsky J, Paluso D, Anderson D, Swan K, Yim JE, Murugesan V, Chindam T, Goraksh N, Alshammari F, Lee H, Trivedi M, Hudlikar AN, Katrak V. The contribution of skin blood flow in warming the skin after the application of local heat; the duality of the Pennes heat equation. Med Eng Phys. 2011;33(3):325–329. doi: 10.1016/j.medengphy.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Seet RC, Lee CY, Loke WM, Huang SH, Huang H, Looi WF, Chew ES, Quek AM, Lim EC, Halliwell B. Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic Biol Med. 2011;50(12):1787–1793. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Whitney RJ. The measurement of changes in human limb-volume by means of a mercury-inrubber strain gauge. J Physiol. 1949;109(1-2) Proc, 5. [PubMed] [Google Scholar]
- 6.Farage MA, Miller KW, Elsner P, Maibach HI. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res. 2008;20(3):195–200. doi: 10.1007/BF03324769. [DOI] [PubMed] [Google Scholar]
- 7.Farage MA, Miller KW, Maibach HI. Textbook of aging skin. Berlin Heidelberg: Springer-Verlag; 2010. [Google Scholar]
- 8.Triggle CR, Ding H. The endothelium in compliance and resistance vessels. Front Biosci (Schol Ed) 2011;3:730–744. doi: 10.2741/s183. [DOI] [PubMed] [Google Scholar]
- 9.Petrofsky JS, LeDonne DM, Rinehart JS, Lind AR. Isometric strength and endurance during the menstrual cycle. Eur J Appl Physiol Occup Physiol. 1976;35(1):1–10. doi: 10.1007/BF00444652. [DOI] [PubMed] [Google Scholar]
- 10.Clarke RS, Hellon RF, Lind AR. Vascular reactions of the human forearm to cold. Clin Sci (Lond) 1958;17(1):165–179. [PubMed] [Google Scholar]
- 11.Haspicova M, Milek D, Siklova-Vitkova M, Wedellova Z, Hejnova J, Bajzova M, Stich V, Polak J. Post-prandial endothelial dysfunction is ameliorated following weight loss in obese premenopausal women. Med Sci Monit. 2011;17(11):CR634–639. doi: 10.12659/MSM.882048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polito MD, da Nóbrega AC, Farinatti P. Blood pressure and forearm blood flow after multiple sets of a resistive exercise for the lower limbs. Blood Press Monit. 2011;16(4):180–185. doi: 10.1097/MBP.0b013e328348cac4. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys PW, Lind AR. The blood flow through active and inactive muscles of the forearm during sustained hand-grip contractions. J Physiol. 1963;166:120–135. doi: 10.1113/jphysiol.1963.sp007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenger CB, Stephenson LA, Durkin MA. Effect of nerve block on response of forearm blood flow to local temperature. J Appl Physiol. 1986;61(1):227–232. doi: 10.1152/jappl.1986.61.1.227. [DOI] [PubMed] [Google Scholar]
- 15.Proctor DN, Halliwill JR, Shen PH, Vlahakis NE, Joyner MJ. Peak calf blood flow estimates are higher with Dohn than with Whitney plethysmograph. J Appl Physiol. 1996;81(3):1418–1422. doi: 10.1152/jappl.1996.81.3.1418. [DOI] [PubMed] [Google Scholar]
- 16.Green S, Thorp R, Reeder EJ, Donnelly J, Fordy G. Venous occlusion plethysmography versus Doppler ultrasound in the assess-ment of leg blood flow during calf exercise. Eur J Appl Physiol. 2011;111(8):1889–1900. doi: 10.1007/s00421-010-1819-6. [DOI] [PubMed] [Google Scholar]
- 17.Cook JS, Sauder CL, Ray CA. Melatonin differentially affects vascular blood flow in humans. Am J Physiol Heart Circ Physiol. 2011;300(2):H670–4. doi: 10.1152/ajpheart.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol. 2011;301(4):H1302–10. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley CJ, Reddy AK, Madala S, Entman ML, Michael LH, Taffet GE. Doppler velocity measurements from large and small arteries of mice. Am J Physiol Heart Circ Physiol. 2011;301(2):H269–78. doi: 10.1152/ajpheart.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26(3):485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 21.Gerhard-Herman M, Gardin JM, Jaff M, Mohler E, Roman M, Naqvi TZ, American Society of Echocardiography Society for Vascular Medicine and Biology. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echo-cardiography and the Society for Vascular Medicine and Biology. Vasc Med. 2006;11(3):183–200. doi: 10.1177/1358863x06070516. [DOI] [PubMed] [Google Scholar]
- 22.Gerhard-Herman M, Gardin JM, Jaff M, Mohler E, Roman M, Naqvi TZ, American Society of Echocardiography Society of Vascular Medicine and Biology. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19(8):955–972. doi: 10.1016/j.echo.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254(5495):56–58. doi: 10.1038/254056a0. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson GE, Tenland T, Obert PA. A new instrument for continuous measurement of tissue blood flow by light beating spectroscopy. IEEE Trans Biomed Eng. 1980;27(1):12–19. doi: 10.1109/TBME.1980.326686. [DOI] [PubMed] [Google Scholar]
- 25.Petrofsky J, Bains G, Prowse M, Gunda S, Berk L, Raju C, Ethiraju G, Vanarasa D, Madani P. Does skin moisture influence the blood flow response to local heat? A re-evaluation of the Pennes model. J Med Eng Technol. 2009;33(7):532–537. doi: 10.1080/03091900902952683. [DOI] [PubMed] [Google Scholar]
- 26.Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009;11(1):39–43. doi: 10.1089/dia.2008.0011. [DOI] [PubMed] [Google Scholar]
- 27.Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and skin blood flow. Med Sci Monit. 2008;14(8):CR399–405. [PubMed] [Google Scholar]
- 28.Tew GA, Klonizakis M, Crank H, Briers JD, Hodges GJ. Comparison of laser speckle contrast imaging with laser Doppler for assessing microvascular function. Microvasc Res. 2011;82(3):326–332. doi: 10.1016/j.mvr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Jakobsson A, Nilsson GE. Prediction of sampling depth and photon pathlength in laser Doppler flowmetry. Med Biol Eng Comput. 1993;31(3):301–307. doi: 10.1007/BF02458050. [DOI] [PubMed] [Google Scholar]
- 30.Fredriksson I, Larsson M, Strömberg T. Measurement depth and volume in laser Doppler flowmetry. Microvasc Res. 2009;78(1):4–13. doi: 10.1016/j.mvr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Fullerton A, Stücker M, Wilhelm KP, Wårdell K, Anderson C, Fischer T, Nilsson GE, Serup J, European Society of Contact Dermatitis Standardization Group Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. A report from the Standardization Group of the European Society of Contact Dermatitis based upon the HIRELADO European community project. Contact Dermatitis. 2002;46(3):129–140. doi: 10.1034/j.1600-0536.2002.460301.x. [DOI] [PubMed] [Google Scholar]
- 32.Petrofsky JS, McLellan K, Prowse M, Bains G, Berk L, Lee S. The effect of body fat, aging, and diabetes on vertical and shear pressure in and under a waist belt and its effect on skin blood flow. Diabetes Technol Ther. 2010;12(2):153–160. doi: 10.1089/dia.2009.0123. [DOI] [PubMed] [Google Scholar]
- 33.Anderson GT, Valvano JW. A small artery heat transfer model for self-heated thermistor measurements of perfusion in the kidney cortex. J Biomech Eng. 1994;116(1):71–78. doi: 10.1115/1.2895707. [DOI] [PubMed] [Google Scholar]
- 34.Hertzman AB, Randall WC. Regional differences in the basal and maximal rates of blood flow in the skin. J Appl Physiol. 1948;1(3):234–241. doi: 10.1152/jappl.1948.1.3.234. [DOI] [PubMed] [Google Scholar]
- 35.Hertzman AB, Randall WC. Further studies on the correlation between skin volume pulses and blood flow. Fed Proc. 1948;7(1 Pt 1):54. [PubMed] [Google Scholar]
- 36.Hertzman AB. Photoelectric plethysmography of the skin. Methods Med Res. 1948;1:177–182. [PubMed] [Google Scholar]
- 37.Davis DL, Hertzman AB. The analysis of vascular reactions in the nasal mucosa with the photoelectric plethysmograph. Ann Otol Rhinol Laryngol. 1957;66(3):622–640. doi: 10.1177/000348945706600302. [DOI] [PubMed] [Google Scholar]
- 38.Simonson E. Photoelectric plethysmography; methods, normal standards, and clinical application. Geriatrics. 1956;11(10):425–433. [PubMed] [Google Scholar]
- 39.Stengele E, Winkler F, Trenk D, Jähnchen E, Petersen J, Roskamm H. Digital pulse plethysmography as a non-invasive method for predicting drug-induced changes in left ventricular preload. Eur J Clin Pharmacol. 1996;50(4):279–282. doi: 10.1007/s002280050108. [DOI] [PubMed] [Google Scholar]
- 40.Drummond PD, Granston A. Facial pain increases nausea and headache during motion sickness in migraine sufferers. Brain. 2004;127(Pt 3):526–534. doi: 10.1093/brain/awh061. [DOI] [PubMed] [Google Scholar]
- 41.Petrofsky JS. In vivo measurement of brain blood flow in the cat. IEEE Trans Biomed Eng. 1979;26(8):441–445. doi: 10.1109/tbme.1979.326568. [DOI] [PubMed] [Google Scholar]
- 42.Halter RJ, Hartov A, Paulsen KD. Imaging forearm blood flow with pulse-ox gated electrical impedance tomography. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1192–1195. doi: 10.1109/IEMBS.2008.4649376. [DOI] [PubMed] [Google Scholar]
- 43.Ward KR, Tiba MH, Draucker GT, Proffitt EK, Barbee RW, Gunnerson KJ, Reynolds PS, Spiess BD. A novel noninvasive impedance-based technique for central venous pressure measurement. Shock. 2010;33(3):269–273. doi: 10.1097/SHK.0b013e3181ab9b9b. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery LD, Dietrich MS, Armer JM, Stewart BR, Ridner SH. Segmental blood flow and hemodynamic state of lymphedematous and nonlymphedematous arms. Lymphat Res Biol. 2011;9(1):31–42. doi: 10.1089/lrb.2010.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cash JD, Lind AR, McNicol GW, Woodfield DG. Fibrinolytic and forearm blood flow responses to intravenous adrenaline in healthy subjects. Life Sci. 1969;8(3):207–213. doi: 10.1016/0024-3205(69)90095-2. [DOI] [PubMed] [Google Scholar]
- 46.Lassen NA, Lindbjerg IF, Dahn I. Validity of the Xenon-133-method for measurement of muscle blood flow evaluated by simultaneous venous occlusion plethysmography: observations in the calf of normal man and in patients with occlusive vascular disease. Circ Res. 1965;16:287–293. doi: 10.1161/01.res.16.3.287. [DOI] [PubMed] [Google Scholar]
- 47.Johansson B, Linder E, Seeman T. Collateral blood flow in the myocardium of dogs measured with krypton. Acta Physiol Scand. 1964;62:263–270. doi: 10.1111/j.1748-1716.1964.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 48.Hohimer AR, Chao CR, Bissonnette JM. The effect of combined hypoxemia and cephalic hypotension on fetal cerebral blood flow and metabolism. J Cereb Blood Flow Metab. 1991;11(1):99–105. doi: 10.1038/jcbfm.1991.11. [DOI] [PubMed] [Google Scholar]
- 49.Jaskille AD, Ramella-Roman JC, Shupp JW, Jordan MH, Jeng JC. Critical review of burn depth assessment techniques: part II. Review of laser doppler technology. J Burn Care Res. 2010;31(1):151–157. doi: 10.1097/BCR.0b013e3181c7ed60. [DOI] [PubMed] [Google Scholar]
- 50.Charkoudian N, Johnson JM. Reflex control of cutaneous vaso-constrictor system is reset by exogenous female reproductive hormones. J Appl Physiol. 1999;87(1):381–385. doi: 10.1152/jappl.1999.87.1.381. [DOI] [PubMed] [Google Scholar]
- 51.Fox RH, Edholm OG. Nervous control of the cutaneous circulation. Br Med Bull. 1963;19:110–114. doi: 10.1093/oxfordjournals.bmb.a070027. [DOI] [PubMed] [Google Scholar]
- 52.Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69(1):154–166. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- 53.Solntseva EI, Bukanova IuV. Decrease of the Ca(2+)-dependent K(+)-current by cyclic guanosine monophosphate does not depend on phosphorylation. Ross Fiziol Zh Im I M Sechenova. 2000;86(10):1337–1345. [PubMed] [Google Scholar]
- 54.Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol. 2009;107(5):1438–1444. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91(4):1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 56.Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and skin blood flow. Med Sci Monit. 2008;14(8):CR399–405. [PubMed] [Google Scholar]
- 57.Petrofsky JS. The effect of type-2-diabetes-related vascular endothelial dysfunction on skin physiology and activities of daily living. J Diabetes Sci Technol. 2011;5(3):657–667. doi: 10.1177/193229681100500319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrofsky J, Goraksh N, Alshammari F, Mohanan M, Soni J, Trivedi M, Lee H, Hudlikar AN, Yang CH, Agilan B, Pai N, Chindam T, Murugesan V, Eun Yim J, Katrak V. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90(10C):40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 60.Ding H, Triggle CR. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: assessing the health of the endothelium. Vasc Health Risk Manag. 2005;1(1):55–71. doi: 10.2147/vhrm.1.1.55.58939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 62.Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85(3):824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 63.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85(3):830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- 64.Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86(4):1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 65.Petrofsky JS, Bains GS, Prowse M, Mc Lellan K, Ethiraju G, Lee S, Gunda S, Lohman E, Schwab E. The influence of age and diabetes on the skin blood flow response to local pressure. Med Sci Monit. 2009;15(7):CR325–31. [PubMed] [Google Scholar]
- 66.Kots AY, Bian K, Murad F. Nitric oxide and cyclic GMP signaling pathway as a focus for drug development. Curr Med Chem. 2011;18(22):3299–3305. doi: 10.2174/092986711796504646. [DOI] [PubMed] [Google Scholar]
- 67.Kapakos G, Bouallegue A, Daou GB, Srivastava AK. Modulatory role of nitric oxide/cGMP system in endothelin-1-induced signaling responses in vascular smooth muscle cells. Curr Cardiol Rev. 2010;6(4):247–254. doi: 10.2174/157340310793566055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Wit C, Boettcher M, Schmidt VJ. Signaling across myoendothelial gap junctions--fact or fiction? Cell Commun Adhes. 2008;15(3):231–245. doi: 10.1080/15419060802440260. [DOI] [PubMed] [Google Scholar]
- 69.Lenasi H, Strucl M. The effect of nitric oxide synthase and cyclo-oxygenase inhibition on cutaneous microvascular reactivity. Eur J Appl Physiol. 2008;103(6):719–726. doi: 10.1007/s00421-008-0769-8. [DOI] [PubMed] [Google Scholar]
- 70.Mochizuki T, Yu S, Katoh T, Aoki K, Sato S. Cardioprotective effect of therapeutic hypothermia at 34°C against ischaemia/reperfusion injury mediated by PI3K and nitric oxide in a rat isolated heart model. Resuscitation. 2012;83(2):238–242. doi: 10.1016/j.resuscitation.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 71.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109(6):687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18(1):R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- 73.Loh K, Merry TL, Galic S, Wu BJ, Watt MJ, Zhang S, Zhang ZY, Neel BG, Tiganis T. T cell protein tyrosine phosphatase (TCPTP) deficiency in muscle does not alter insulin signalling and glucose homeostasis in mice. Diabetologia. 2012;55(2):468–478. doi: 10.1007/s00125-011-2386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshida T, Gong J, Xu Z, Wei Y, Duh EJ. Inhibition of pathological retinal angiogenesis by the integrin αvβ3 antagonist tetraiodothyroacetic acid (tetrac) Exp Eye Res. 2012;94(1):41–48. doi: 10.1016/j.exer.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilkin JK, Fortner G. Cutaneous vascular sensitivity to lower aliphatic alcohols and aldehydes in Orientals. Alcohol Clin Exp Res. 1985;9(6):522–525. doi: 10.1111/j.1530-0277.1985.tb05596.x. [DOI] [PubMed] [Google Scholar]
- 76.McLellan K, Petrofsky JS, Zimmerman G, Lohman E, Prowse M, Schwab E, Lee S. The influence of environmental temperature on the response of the skin to local pressure: the impact of aging and diabetes. Diabetes Technol Ther. 2009;11(12):791–798. doi: 10.1089/dia.2009.0097. [DOI] [PubMed] [Google Scholar]
- 77.Petrofsky JS, Alshahmmari F, Lee H, Hamdan A, Yim JE, Shetye G, Neupane S, Somanaboina K, Pathak K, Shenoy S, Dave B, Cho S, Chen WT, Nevgi B, Moniz H, Alshaharani M, Malthane S, Desai R. Reduced endothelial function in the skin in Southeast Asians compared to Caucasians. Med Sci Monit. 2012;18(1):CR1–8. doi: 10.12659/MSM.882185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrofsky J, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the forearm; the impact of rosiglitazone. BMC Endocr Disord. 2005;5(1):4. doi: 10.1186/1472-6823-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- 80.Taylor NA, Allsopp NK, Parkes DG. Preferred room temperature of young vs aged males: the influence of thermal sensation, thermal comfort, and affect. J Gerontol A Biol Sci Med Sci. 1995;50(4):M216–21. doi: 10.1093/gerona/50a.4.m216. [DOI] [PubMed] [Google Scholar]
- 81.Petrofsky J, Bains G, Prowse M, Gunda S, Berk L, Raju C, Ethiraju G, Vanarasa D, Madani P. Does skin moisture influence the blood flow response to local heat? A re-evaluation of the Pennes model. J Med Eng Technol. 2009;33(7):532–537. doi: 10.1080/03091900902952683. [DOI] [PubMed] [Google Scholar]
- 82.Wissler EH. Pennes' 1948 paper revisited. J Appl Physiol. 1998;85(1):35–41. doi: 10.1152/jappl.1998.85.1.35. [DOI] [PubMed] [Google Scholar]
- 83.Almalty AM, Petrofsky JS, Al-Naami B, Al-Nabulsi J. An effective method for skin blood flow measurement using local heat combined with electrical stimulation. J Med Eng Technol. 2009;33(8):663–669. doi: 10.3109/03091900903271646. [DOI] [PubMed] [Google Scholar]
- 84.Evans E, Rendell M, Bartek J, Connor S, Bamisedun O, Dovgan D, Giitter M. Thermally-induced cutaneous vasodilatation in aging. J Gerontol. 1993;48(2):M53–7. doi: 10.1093/geronj/48.2.m53. [DOI] [PubMed] [Google Scholar]
- 85.Malmberg AB, Bley KR. Turning up the heat on pain: TRPV1 receptors in pain and inflammation. Heidelberg: Springer Science; 2005. [Google Scholar]
- 86.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284(5):H1662–7. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 87.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93(5):1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 88.Weiss M, Milman B, Rosen B, Eisenstein Z, Zimlichman R. Analysis of the diminished skin perfusion in elderly people by laser Doppler flowmetry. Age Ageing. 1992;21(4):237–241. doi: 10.1093/ageing/21.4.237. [DOI] [PubMed] [Google Scholar]
- 89.Petrofsky J, Paluso D, Anderson D, Swan K, Alshammari F, Katrak V, Murugesan V, Hudlikar AN, Chindam T, Trivedi M, Lee H, Goraksh N, Yim JE. The ability of different areas of the skin to absorb heat from a locally applied heat source: the impact of diabetes. Diabetes Technol Ther. 2011;13(3):365–372. doi: 10.1089/dia.2010.0161. [DOI] [PubMed] [Google Scholar]
- 90.Lawson D, Petrofsky JS. A randomized control study on the effect of biphasic electrical stimulation in a warm room on skin blood flow and healing rates in chronic wounds of patients with and without diabetes. Med Sci Monit. 2007;13(6):CR258–63. [PubMed] [Google Scholar]
- 91.Petrofsky J, Bains G, Prowse M, Gunda S, Berk L, Raju C, Ethiraju G, Vanarasa D, Madani P. Dry heat, moist heat and body fat: are heating modalities really effective in people who are overweight? J Med Eng Technol. 2009;33(5):361–369. doi: 10.1080/03091900802355508. [DOI] [PubMed] [Google Scholar]
- 92.Petrofsky JS, Bains G, Raju C, Lohman E, Berk L, Prowse M, Gunda S, Madani P, Batt J. The effect of the moisture content of a local heat source on the blood flow response of the skin. Arch Dermatol Res. 2009;301(8):581–585. doi: 10.1007/s00403-009-0957-3. [DOI] [PubMed] [Google Scholar]
- 93.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133(2):159–176. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, Pala MG, Formica F, Paolini G, Catapano AL, Bosi E, Alfieri O, Piatti P. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism. 2009;58(9):1270–1276. doi: 10.1016/j.metabol.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 95.Farage M, Miller K, Maibach H. Text book of ageing skin. Berlin: Springer; 2010. Influence of race, gender, age and diabetes on the skin circluation. [Google Scholar]
- 96.Sacheck JM, Blumberg JB. Role of vitamin E and oxidative stress in exercise. Nutrition. 2001;17(10):809–814. doi: 10.1016/s0899-9007(01)00639-6. [DOI] [PubMed] [Google Scholar]
- 97.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6(10):964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 99.Petrofsky J, Hinds CM, Batt J, Prowse M, Suh HJ. The interrelationships between electrical stimulation, the environment surrounding the vascular endothelial cells of the skin, and the role of nitric oxide in mediating the blood flow response to electrical stimulation. Med Sci Monit. 2007;13(9):CR391–397. [PubMed] [Google Scholar]
- 100.Sen CK, Packer L, Hänninen O. Handbook of oxidants and antioxidants in exercise. 1st ed. Amsterdam: Elsevier; 2000. [Google Scholar]
- 101.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1-2):41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 102.Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847(2):185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 103.Mah E, Matos MD, Kawiecki D, Ballard K, Guo Y, Volek JS, Bruno RS. Vitamin C status is related to proinflammatory responses and impaired vascular endothelial function in healthy, college-aged lean and obese men. J Am Diet Assoc. 2011;111(5):737–743. doi: 10.1016/j.jada.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 104.Rokitzki L, Logemann E, Sagredos AN, Murphy M, Wetzel-Roth W, Keul J. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiol Scand. 1994;151(2):149–158. doi: 10.1111/j.1748-1716.1994.tb09732.x. [DOI] [PubMed] [Google Scholar]
- 105.Rokitzki L, Sagredos AN, Reuss F, Büchner M, Keul J. Acute changes in vitamin B6 status in endurance athletes before and after a marathon. Int J Sport Nutr. 1994;4(2):154–165. doi: 10.1123/ijsn.4.2.154. [DOI] [PubMed] [Google Scholar]
- 106.Neunteufl T, Priglinger U, Heher S, Zehetgruber M, Söregi G, Lehr S, Huber K, Maurer G, Weidinger F, Kostner K. Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. J Am Coll Cardiol. 2000;35(2):277–283. doi: 10.1016/s0735-1097(99)00542-2. [DOI] [PubMed] [Google Scholar]
- 107.Punithavathi VR, Stanely Mainzen Prince P, Kumar MR, Selvakumari CJ. Protective effects of gallic acid on hepatic lipid peroxide metabolism, glycoprotein components and lipids in streptozotocin-induced type II diabetic wistar rats. J Biochem Mol Toxicol. 2011;25(2):68–76. doi: 10.1002/jbt.20360. [DOI] [PubMed] [Google Scholar]
- 108.Punithavathi VR, Stanely Mainzen Prince P. The cardioprotective effects of a combination of quercetin and a-tocopherol on isoproterenol-induced myocardial infarcted rats. J Biochem Mol Toxicol. 2011;25(1):28–40. doi: 10.1002/jbt.20357. [DOI] [PubMed] [Google Scholar]
- 109.Traber MG, Stevens JF. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barker T, Martins TB, Hill HR, Kjeldsberg CR, Trawick RH, Leonard SW, Walker JA, Traber MG. Vitamins E and C modulate the association between reciprocally regulated cytokines after an anterior cruciate ligament injury and surgery. Am J Phys Med Rehabil. 2011;90(8):638–647. doi: 10.1097/PHM.0b013e318214e886. [DOI] [PubMed] [Google Scholar]
- 111.Sies H. Relationship between free radicals and vitamins: an overview. Int J Vitam Nutr Res Suppl. 1989;30:215–223. [PubMed] [Google Scholar]
- 112.Petrofsky J, Laymon MHAN. The effect of multivitamins and coenzyme Q-10 on endothelial function in a young population. Anatom Physiol. 2011;1(1):101. [Google Scholar]
- 113.Pemberton TJ, Mehta NU, Witonsky D, Di Rienzo A, Allayee H, Conti DV, Patel PI. Prevalence of common disease-associated variants in Asian Indians. BMC Genet. 2008;9:13. doi: 10.1186/1471-2156-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bui C, Petrofsky J, Berk L, Shavlik D, Remigio W, Montgomery S. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41(2):490–500. [PMC free article] [PubMed] [Google Scholar]
- 115.Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N. The effect of polymorphism in the intestinal fatty acid-binding protein 2 gene on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6(1):45–49. doi: 10.1111/j.1463-1326.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 116.Yim J, Petrofsky JS, Berk L, Daher N, Lohman E. doi: 10.12659/MSM.882902. Differences in endothelial function between Korean-Asians and Caucasians. Med Sci Monit. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]


