Abstract
Background
This study was conducted to investigate type 2 diabetes mellitus (T2DM) patient perceptions of their pen injectors and determine which features were deemed most important to overall satisfaction.
Methods
Frost & Sullivan conducted a Web-based survey of T2DM patients in the United States in November 2010. Survey participants were initially screened prior to full participation. A total of 1002 adult T2DM patients who were using a pen injector on a regular basis to administer their diabetes medication(s) were surveyed. The survey consisted of 24 questions focused on awareness and current usage of pen injectors by type and brand, specific features of pen injectors, and patients' preferences for and satisfaction with pen injectors.
Results
The majority of surveyed patients were using prefilled pen injectors as compared with durable pens. The LANTUS SoloSTAR (sanofi-aventis) was reported to be the most commonly used pen. The LANTUS SoloSTAR was also ranked highly for overall satisfaction and likelihood of continued usage. Regardless of brand, most surveyed patients reported that they were likely to continue using their current pen. In general, the single most important feature for user satisfaction was an easy push-button injection.
Conclusions
Ease of self-administration is of highest priority to users of pen injectors. Important features facilitating ease of use, such as an easily depressed push-button injection, are likely to minimize the burden on T2DM patients, thereby improving compliance and clinical outcomes.
Keywords: diabetes, injector, insulin, pen
Introduction
Type 2 diabetes mellitus (T2DM) is a disease of considerable burden on patients and their caregivers, particularly for those in the more advanced stages who must rely on injectable therapies such as insulin and glucagon-like peptide-1 analogs to manage their disease. In light of the increasing prevalence of diabetes, along with the aging population and recommendations for earlier initiation of insulin therapy,1 injectable therapies will likely be increasingly relied on as beneficial therapeutics for the management of this disease.
Management of diabetes is undertaken with the goal of preventing or minimizing organ damage and other acute and long-term complications. Patient adherence to a prescribed therapeutic regimen to manage their diabetes is of utmost importance in ensuring optimal clinical outcomes. Persuading patients to adhere to chronic therapy that is vital for disease management, but offers no relief of symptoms, may be challenging. When self-injection is a component of that therapy, compliance can become even more of a challenge. The introduction of pen injectors for injectable therapeutics, such as insulin, was a significant advancement in diabetes management, greatly facilitating the ease of self-injection. Because patient compliance is critical to realizing the clinical benefits of therapy, the injection device that these products are marketed with may be an important consideration in patient and physician choice of therapy. Therefore, Frost & Sullivan conducted a patient survey in order to investigate patient usage patterns and perceptions of marketed pen injectors.
Methods
Participants
Frost & Sullivan conducted a Web-based survey of T2DM patients in the United States in November 2010. Survey participants were initially screened for eligibility prior to full participation. Screening questions queried potential survey participants regarding demographic information, diagnosis of diabetes with regular use of antidiabetic medication, and current use of a pen injector. The targeted sample was 1000 adult patients with type 2 diabetes, with equal distribution across the four regions of the United States (Northeast, South, Midwest, and West). Both male and female adult patients were targeted; however, sample quotas were established for age and gender. Specifically, a greater emphasis was placed on targeting men (approximately 60%, versus 40% women) and older patients (approximately 70% older than 70 years, and 30% younger than 40 years). Patients of any race/ethnicity were included in the targeted sample.
Survey Implementation
This survey was implemented by WorldOne, a global health care data collection firm, and was fielded during the period of November 15, 2010, to December 15, 2010. Respondents were recruited from multiple sources throughout the Web using a variety of techniques and campaigns designed to attract a wide variety of potential respondents. In exchange for completing the survey, participants were eligible to receive a variety of rewards such as sweepstakes points, charity donations, points for gift cards, music downloads, loyalty points such as airline miles, and other similar incentives. Verification of geographical location via IP address was used, as is standard for online panel studies. Although every effort was made to reduce sample bias, we acknowledge that it is not possible to control all variables that may cause a possible bias.
Survey Instrument
The online survey consisted of a total of 24 multiple-choice, yes/no, and differential-scale questions. Survey questions were designed to assess awareness of pen injectors for diabetes patients, measure current usage and importance of features/attributes, and assess end user demand for pen injectors. A select number of pen injector brands and features intended to capture a significant portion of the market were included in this survey. Brands of pen injectors assessed in this survey are outlined in Table 1.
Table 1.
Pen Injectors Included in This Survey
| Autopen (Owen Mumford USA Ltd., Marietta, GA) | FlexPen (Novo Nordisk, Bagsvaerd, Denmark) |
| BYETTA pen (Amylin Pharmaceuticals, San Diego, CA) | NovoPen Junior (Novo Nordisk, Bagsvaerd, Denmark) |
| DIAPEN (Haselmeier GmbH, Stuttgart, Germany) | NovoPen 3 (Novo Nordisk, Bagsvaerd, Denmark) |
| Humalog KwikPen (Eli Lilly, Indianapolis, IN) | NovoPen 3 PenMate (Novo Nordisk, Bagsvaerd, Denmark) |
| HumaPen LUXURA (Eli Lilly, Indianapolis, IN) | NovoPen 4 (Novo Nordisk, Bagsvaerd, Denmark) |
| HumaPen MEMOIR (Eli Lilly, Indianapolis, IN) | OptiClik pen (Ypsomed, Burgdorf, Switzerland) |
| Humulin pen (Eli Lilly, Indianapolis, IN) | OptiPen Pro (Ypsomed, Burgdorf, Switzerland) |
| InDuo (LifeScan, a Johnson & Johnson company, Milpitas, CA, and Novo Nordisk, Bagsvaerd, Denmark) | OptiSet (Ypsomed, Burgdorf, Switzerland) |
| Lantus SoloSTAR Pen (sanofi-aventis, Bridgewater, NJ) | SymlinPen (Amylin Pharmaceuticals, San Diego, CA) |
| Novolin InnoLet (Novo Nordisk, Bagsvaerd, Denmark) | |
Data Analysis
Survey responses were tallied and analyzed for statistical significance across demographic parameters. Specifically, equality of means (assuming equal variance) was analyzed using the independent t-test at the 95% confidence level. Equality of percentages was analyzed using the independent z-test at the 95% confidence level.
Results
Response Rate
A total of 1002 adult T2DM patients (543 men and 459 women) from across the United States who use a pen injector on a regular basis to deliver medication to manage their disease were selected from 15,699 potential participants who initially responded to this survey. Of the total potential respondent pool, 50.2% (7873) did not meet the inclusion criteria and were disqualified. Reasons for disqualification included geographic location outside the United States, under 18 years of age, diabetes type, diabetes medication delivery method, and no knowledge of the manufacturer of their injection pen (13, 14, 954, 6572, and 320 respondents, respectively). An additional 43.5% (6824) did not complete the survey. The fielding period for this survey was 1 month, from November 15, 2010, through December 15, 2010.
Demographics
Demographic information for qualified respondents is provided in Table 2. More than half of the participants (57%) were aged 50 years or older. Distribution across the geographic regions was approximately equal, with a slightly higher proportion (37%) residing in the Southern United States and a smaller proportion (18%) residing in the Northeastern United States. Most of the participants (82%) identified themselves as Caucasian/white, with the remainder distributed approximately equally between African-American (6%), Hispanic/Latino (6%), and Asian-American (3%). Ninety-three percent of the participants reported having medical insurance, which is above the national average of 83.7%.2
Table 2.
Demographic Characteristics of Survey Participants (N = 1002)
| Gender | |||
|---|---|---|---|
| N (%) | |||
| Male | 543 (54) | ||
| Female | 459 (46) | ||
| Age group by gender | |||
|---|---|---|---|
| N (%) | |||
| Age group | Total sample | Males | Females |
| 18–29 | 75 (7) | 31 (6) | 44 (10)a |
| 30–39 | 226 (23) | 100 (18) | 126 (27)a |
| 40–49 | 134 (13) | 77 (14) | 57 (12) |
| 50–59 | 248 (25) | 133 (24) | 115 (25) |
| 60–69 | 261 (26) | 165 (30)b | 96 (21) |
| 70+ | 58 (6) | 37 (7) | 21 (5) |
| Geographic distribution | |||
|---|---|---|---|
| N (%) | |||
| Region | Total sample | Males | Females |
| Northeastern U.S. | 180 (18) | 107 (20) | 73 (16) |
| Southern U.S. | 371 (37) | 184 (34) | 187 (41)a |
| Midwestern U.S. | 221 (22) | 119 (22) | 102 (22) |
| Western U.S. | 230 (23) | 133 (24) | 97 (21) |
| Race/ethnicity | |||
|---|---|---|---|
| N (%) | |||
| Racial/ethnic background | Total sample | Males | Females |
| Caucasian/white | 819 (82) | 463 (85) | 356 (78) |
| African-American | 61 (6) | 20 (4) | 41 (9)a |
| Hispanic/Latino | 62 (6) | 33 (6) | 29 (6) |
| Asian-American | 35 (3) | 18 (3) | 17 (4) |
| Pacific Islander | 4 (<1) | 1 (<1) | 3 (1) |
| Medical insurance coverage | |||
|---|---|---|---|
| N (%) | |||
| Yes | 933 (93) | 512 (94) | 421 (92) |
| No | 69 (7) | 31 (6) | 38 (8) |
Statistically higher versus males at the 95% confidence level.
Statistically higher versus females at the 95% confidence level.
Pen Injector Usage
Frequency of pen injector use and prevalence of self-injection is summarized in Table 3. Frequency of daily injections was reported to be once (34%) or twice (31%) per day by the majority of participants, with 17% and 14% reporting using their pen three times daily and four times daily, respectively. As expected, more frequent injection was associated with increasing age. This is presumed to be a reflection of a higher prevalence of advanced disease state and the associated requirement of more intensive insulin therapy in this age group (data not shown). Nearly all participants (96%) reported that their injectable diabetes medications were administered via self-injection, independent of gender or age. While the majority of participants reported performing self-injection, a small proportion (4%) responded that their injectable medication was administered by another member of the household, a nurse, or other health care personnel.
Table 3.
Usage Patterns of Pen Injectors
| Frequency of daily injections | |
|---|---|
| Number of injections per day | Total sample |
| N (%) | |
| One | 337 (34) |
| Two | 314 (31) |
| Three | 168 (17) |
| Four | 136 (14) |
| Other | 47 (5) |
| Self-injection versus caregiver injection | |
|---|---|
| Giver of injection | Total sample |
| N (%) | |
| Self | 960 (96) |
| Another household member | 25 (2) |
| Nurse or other health care personnel | 7 (<1) |
| Other | 10 (1) |
Usage of pen injectors by type (prefilled disposable versus durable) is presented in Figures 1 and 2. The majority of survey participants (80%) reported using a prefilled disposable pen compared with 20% who reported using a durable pen. Usage of prefilled disposable pens was highest among women (83% women versus 77% men; p < .05; Figure 1) and patients aged 40 years and older (88% versus 60% aged 18 to 39 years; p < .05; Figure 2).
Figure 1.
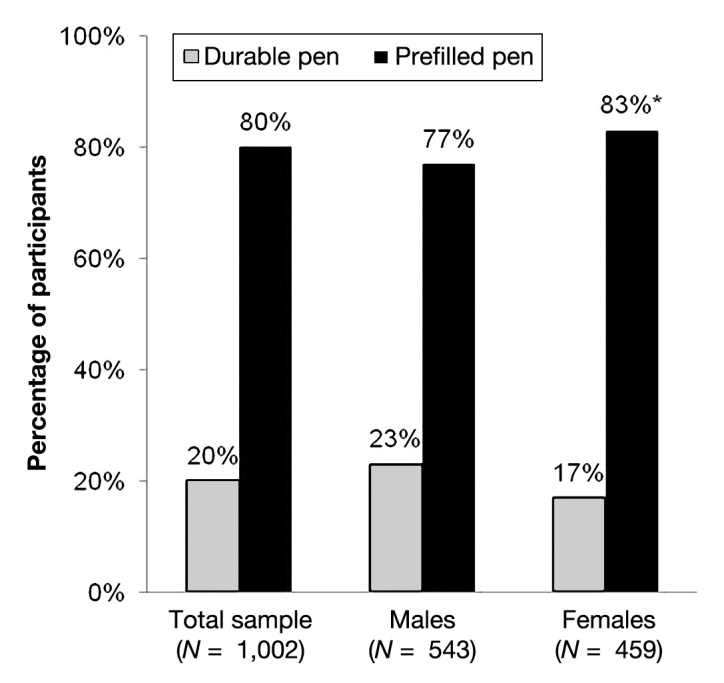
Usage of types of injection pens by gender. The asterisk indicates statistical isgnificance versus males (p < .05).
Figure 2.
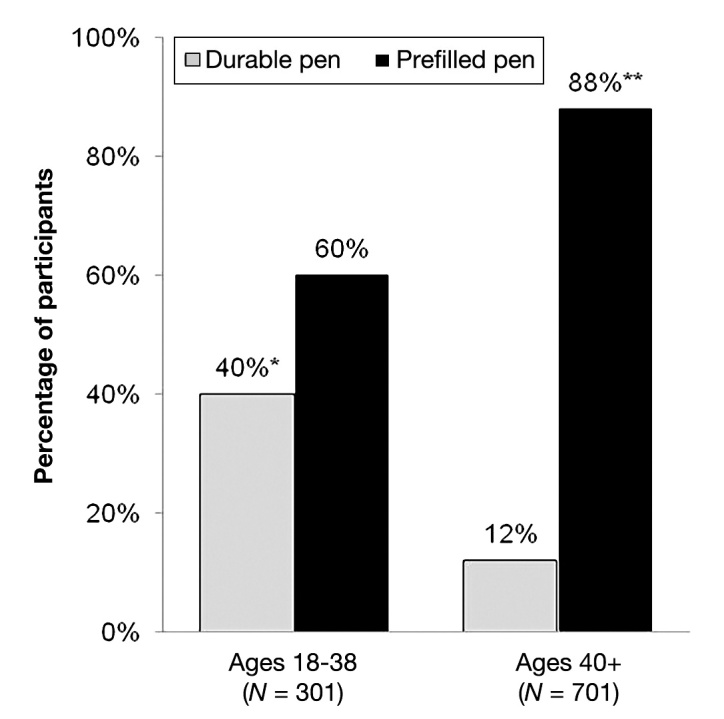
Usage of types of injection pens by age group. The single asterisk indicates statistical significance versus age 40+ years (p < .05). The double asterisks indicate statistical significance versus age 18–39 (p < .05).
The popularity and brand awareness of pen injectors is summarized in Figures 3 and 4. The most predominant brand of pen injector was the Lantus SoloSTAR, currently used by 26% (N = 265) of the participants. The FlexPen, used by 18% of the participants, was the second most popular pen with regard to current use but received the highest score for aided brand recognition. The Byetta pen also received high scores for aided brand awareness (46%), although it was being used by only 14% of participants. The majority of the five least recalled brands of pen injectors (Figure 4) were unavailable in the United States at the time of this survey; however, the level of awareness present, albeit relatively low, could be a reflection of awareness generated by the Internet or recent emigration to the United States by some of the respondents.
Figure 3.
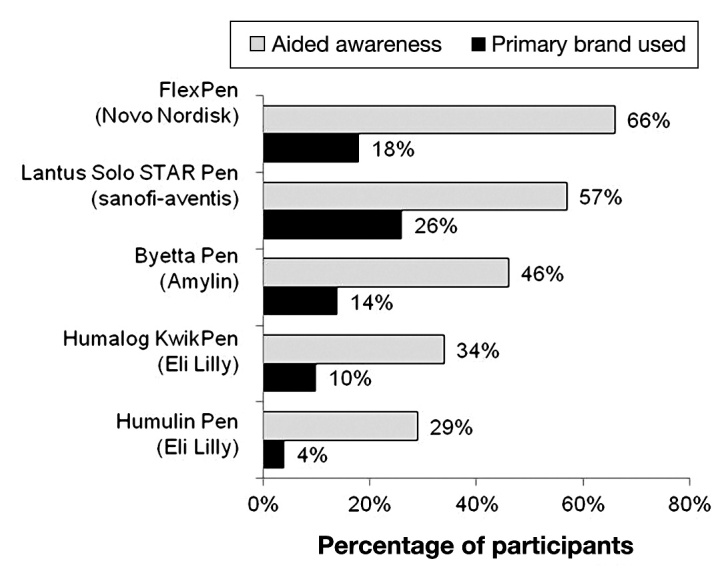
Top five injection pen brands recalled and proportion used as primary brand (N = 1002).
Figure 4.
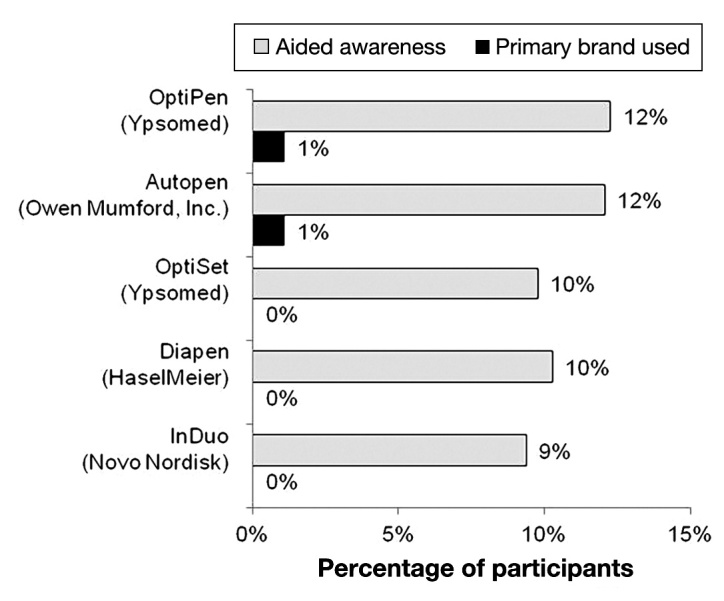
Bottom five injection pen brands recalled and proportion used as primary brand (N = 1002).
Pen Injector Features
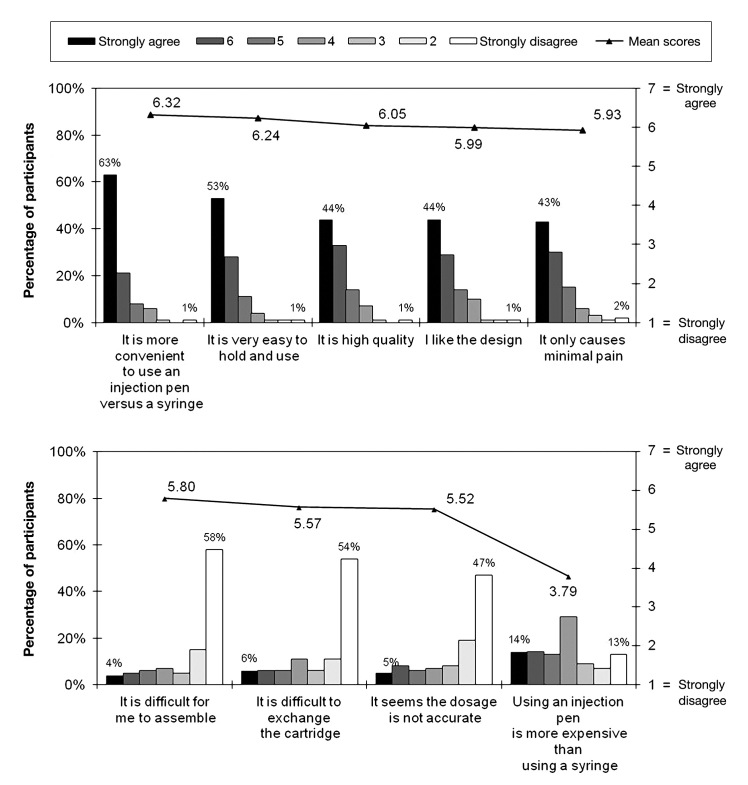
Survey responses to overall perceptions of pen injectors are presented in Figure 5. When asked about their overall perceptions of pen injectors, regardless of brand, the majority of participants (63%) felt strongly that pen injectors were more convenient than syringes and that their pen injectors were easy to hold and use (53%). Feelings toward the overall quality and design were also positive, albeit slightly less skewed. The majority of surveyed patients strongly disagreed that their pen injectors were difficult to assemble (58%) or that it was difficult to exchange the cartridge (54%). Participants were largely neutral in their perception of the relative expense of using a pen injector, with the majority neither agreeing nor disagreeing with the statement “Using an injection pen is more expensive than using a syringe.” Generally speaking, when demographic comparisons were made, females and older patients tended to be more positive than their counterparts (data not shown).
Figure 5.
Beliefs toward injection pens. Participants responded to a series of questions assessing their beliefs toward injection pens. Level of agreement or disagreement was assessed using a 7-point scale.
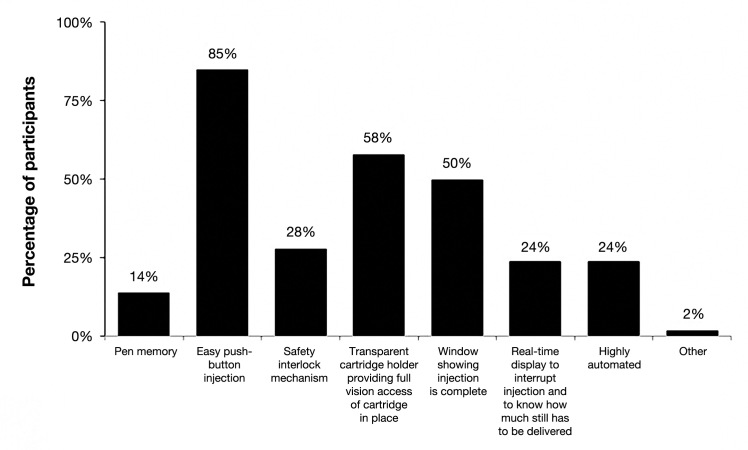
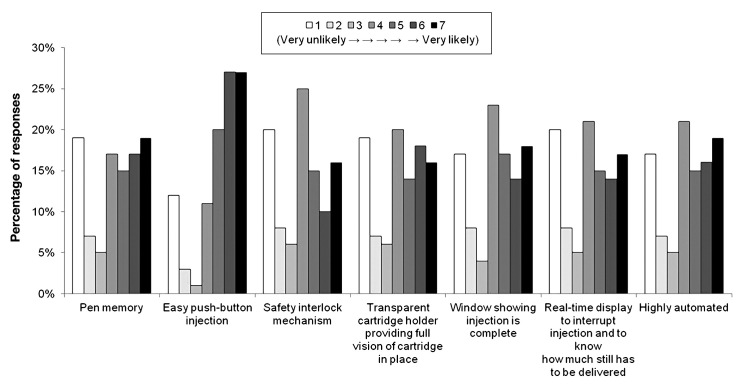
Figures 6 and 7 depict survey participant responses to questions assessing features included in their current pen injectors and the likelihood of their switching to another brand of pen injector for features not included in their current pens. When asked about which features their current pen injector includes, 85% of participants identified “easy push-button injection.” Other common features according to participants were a “transparent cartridge holder providing full vision access of cartridge in place” (58%) and a “window showing injection is complete” (50%) (Figure 6). When asked about the features not included in their current pen for which they would be willing to switch brands and the likelihood of their switching, “easy push-button injection” received the largest proportion of “very likely” responses (27%, N = 147) as well as the lowest proportion of “very unlikely” responses (12%, N = 17). For all remaining features, most participants were neutral or roughly divided regarding the likelihood of switching brands (Figure 7).
Figure 6.
Features of current injection pens (N = 1002). Participants were asked to identify the features included in their current injection pens. Participants were allowed to choose multiple features.
Figure 7.
Features for which participants were willing to switch brands. Participants were asked to reveal their willingness to switch to another injection pen if their pen did not include these features. Responses were recorded on a 7-point scale ranging from “very unlikely” to “very likely.”
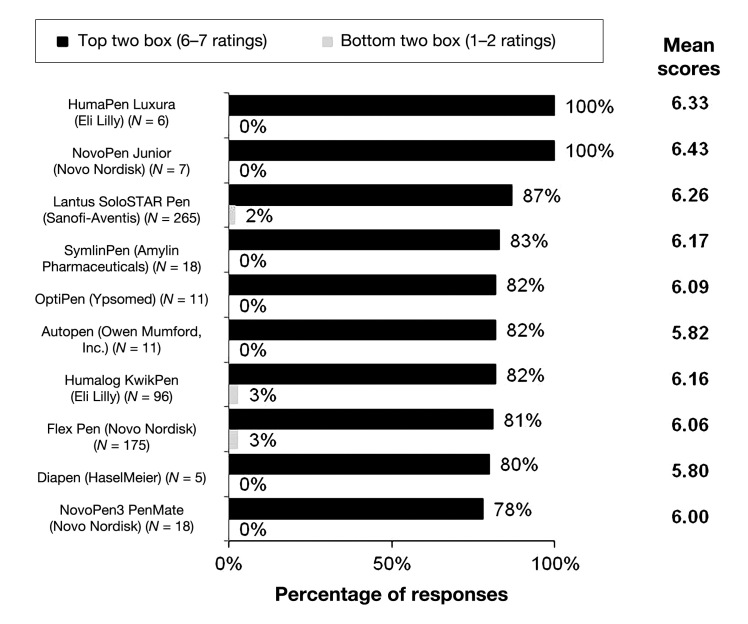
Figure 8 depicts the top 10 brands of pen injectors by satisfaction ratings. Pen injector brands were ranked according to the percentage of top two box (6–7) satisfaction ratings received on a scale of 1 to 7, with 7 representing “very satisfied” and 1 representing “very dissatisfied.” When participants were asked how satisfied they were with the pen injector brand that they were currently using, the brands receiving the highest scores for satisfaction were the NovoPen Junior and the HumaPen Luxura, with 100% of top two box satisfaction scores and average scores of 6.43 (N = 7) and 6.33 (N = 6), respectively. The Lantus SoloSTAR scored the third highest, with 87% (N = 265) of top two box satisfaction scores and a mean score of 6.26 out of 7 points. Among the top 10 brands, top two box satisfaction scores ranged from 100% to 78%, with mean scores ranging from 6.43 to 5.80 out of 7 points. The top three reasons for high satisfaction with pen injectors were easiest to self administer (36%), dose accuracy (18%), and no (or minimal) discomfort/pain (11%; data not shown). Overall, 84% of surveyed patients (841) reported that they were likely to continue using their current pen (top two box ratings; data not shown).
Figure 8.
Top 10 injection pen brands by satisfaction (N = 1002). Participants were asked to gauge their level of satisfaction with their current brand of injection pen brand. Level of satisfaction was based on a 7-point scale, where 7 represents “very satisfied” and 1 represents “very dissatisfied.” Brands were ranked according to percentage of top two box (6-7) ratings.
Discussion
This survey was undertaken to evaluate T2DM patient satisfaction with their pen injectors used in their disease management regimens and to investigate which features were deemed most desirable. According to the results of this survey, the single most important pen injector feature for patient satisfaction is easy push-button injection. This was not only the most commonly recalled feature of current pen injectors, but also the only feature for which patients were clearly likely to switch brands if their pen did not include this feature. Interestingly, one of the pen injector brands that received the highest score for patient satisfaction was the NovoPen Junior, a pen designed for children. Although this pen was reportedly used by only a few participants (N = 7), those patients report high satisfaction with the NovoPen Junior, presumably because it makes the self-injection process easier for adults as well as children.
The Lantus SoloSTAR was reported to be the most commonly used pen, as well as the pen with the highest brand recognition. This is likely a direct reflection of Lantus being the number one prescribed branded insulin in the United States (based on TRX data from IMS Health, NPA monthly database, time period from May 2003 to March 2010), although this insulin brand's popularity may be at least partially due to the desirable SoloSTAR pen delivery option, which received the highest scores for satisfaction when corrected for number of users.
Significantly more uninsured patients in this survey were aged 40 years and above (8% versus 4% aged 18–39 years; p < .05). However, surprisingly, this age group was significantly less likely than the 18–39 years age group to perceive injection pens as being more expensive to use versus a syringe. Although details of individual insurance plans are not known, it is possible that, in general, insurance coverage of the age 40 years and above group favors coverage of injection pens more than the 18–39 year age group.
Limitations of this study include unequal distribution and inadequate number of users of some of the pen injector brands to draw statistically meaningful conclusions, particularly regarding feature preferences, and possible selection bias due to the use of an online survey methodology.
Conclusions
Type 2 diabetes patients who use injectable medication to manage their disease appear to be primarily concerned with the ease of performing their injections and do not express great desire for advanced features in their pen injectors. Pens that make self-injection as simple and painless as possible without complicating the process, particularly for older patients, will likely be well received by users. The ideal combination of an effective and safe injectable medication with the convenience of an easy-to-use pen injector may help improve patient compliance and overall clinical outcomes.
Acknowledgments
The authors thank WorldOne for conducting the interviews. Portions of these data have been published for subscribers of Frost.com and in Drug Development and Delivery, June 2011.
Glossary
Abbreviations
- (T2DM)
type 2 diabetes mellitus
Funding
This study was supported by Frost & Sullivan.
Disclosure
Deborah Toscano, Jennifer Brice, and Christina Alfaro are employees of Frost & Sullivan.
References
- 1.American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeNavas-Walt C, Proctor BD, Smith JC, U.S. Census Bureau. Current population reports, P60-239 . Washington DC: U.S. Government Printing Office; 2011. Income, poverty, and health insurance coverage in the United States: 2010. [Google Scholar]