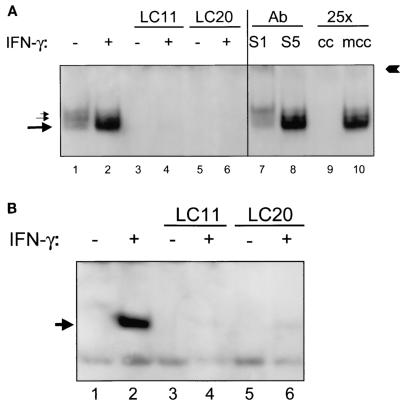
FIG. 4.
H. pylori decreases IFN-γ-induced DNA binding to STAT1 in epithelial cells. Confluent MKN45 and HEp-2 cells were serum starved overnight, infected with H. pylori strains LC11 and LC20, rinsed, and stimulated with IFN-γ. Equal amounts of nuclear protein extracts were then analyzed by EMSA. (A) MKN45 cells exhibited constitutive DNA binding to STAT1 (lower left arrow) and STAT3 (upper left arrow) homodimers and to STAT1-STAT3 (middle left arrow) heterodimers (lane 1). DNA binding to STAT1 homodimers was increased following stimulation with IFN-γ (1 ng/ml, 30 min) (lane 2). Infection with H. pylori strains LC11 and LC20 (MOI, 100:1; 6 h) decreased constitutive (lanes 3 and 5) and IFN-γ induced (lanes 4 and 6) DNA binding to STAT1 (n = 3). The identity of the band as STAT1 was confirmed by supershift of the band with a STAT1 (S1) antibody (Ab) (right arrow) but not with a STAT5 (S5) antibody. The specificity of the radiolabeled double-stranded DNA oligonucleotide was confirmed by competition of the band with a nonradiolabeled STAT1 (25× molar excess) cold competitor (cc) but not with a STAT6 cold competitor (mcc). (B) HEp-2 cells demonstrated inducible STAT1 DNA binding in response to stimulation with IFN-γ (20 ng/ml, 30 min) (arrow, lane 2). Infection with H. pylori strains LC11 and LC20 did not induce STAT1 DNA binding (lanes 3 and 5). IFN-γ-induced DNA binding to STAT1 was decreased by infection with LC11 and LC20 (lanes 4 and 6) (n = 3).