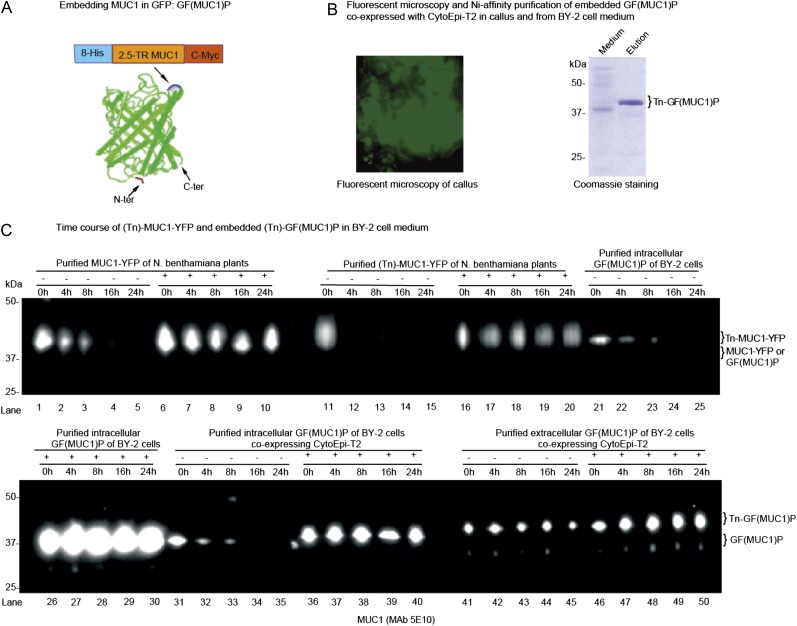
Figure 4.
O-Glycosylation and embedding in GFP stabilize MUC1 in BY-2 cell culture. A, Construct design for embedding MUC1-2.5TR into GFP [GF(MUC1)P], and corresponding barrel structure showing the loop into which MUC1-2.5 TR flanked with His8 and C-Myc tags was inserted after Asp-196 in the loop between the β-strands (blue) located opposite the N and C termini. This figure was adapted from Kobayashi et al. (2008). B, Fluorescence microscopy of a stable BY-2 transgenic line coexpressing GF(MUC1)P with the O-glycosylation machinery CytoEpi-T2 (left panel), and SDS-PAGE Coomassie blue staining of nickel chromatography-purified secreted GalNAc-glycosylated GF(MUC1)P [Tn-GF(MUC1)P] of this line (right panel). C, Analysis of the degradation of MUC1-YFP and GF(MUC1)P in BY-2 cell culture medium by SDS-PAGE western blotting: (1) MUC1-YFP (lanes 1–10) and GalNAc-glycosylated MUC1-YFP (Tn-MUC1-YFP; lanes 11–20) transiently produced in leaves of N. benthamiana plants; (2) intracellularly embedded GF(MUC1)P (lanes 21–30) purified from a stably transformed BY-2 cell line expressing GF(MUC1)P alone; (3) intracellularly (lanes 31–40) and extracellularly (lanes 41–50) embedded (Tn)-GF(MUC1)P purified from a transgenic BY-2 cell line additionally coexpressing CytoEpi-T2. The isolated proteins were added to 7-d-old unboiled (−) or boiled (+) wild-type BY-2 medium fractions, which were further incubated for up to 24 h under the same conditions. Approximately 5 μg of purified proteins was added to 1 mL of BY-2 medium fractions, of which approximately 15 μL was loaded and blots probed with anti-MUC1 MAb 5E10. The corresponding Tn-MUC1-specific western blot using MAb 5E5 is presented in Supplemental Figure S3. [See online article for color version of this figure.]