Figure 5. Dimerization in E. coli membranes.
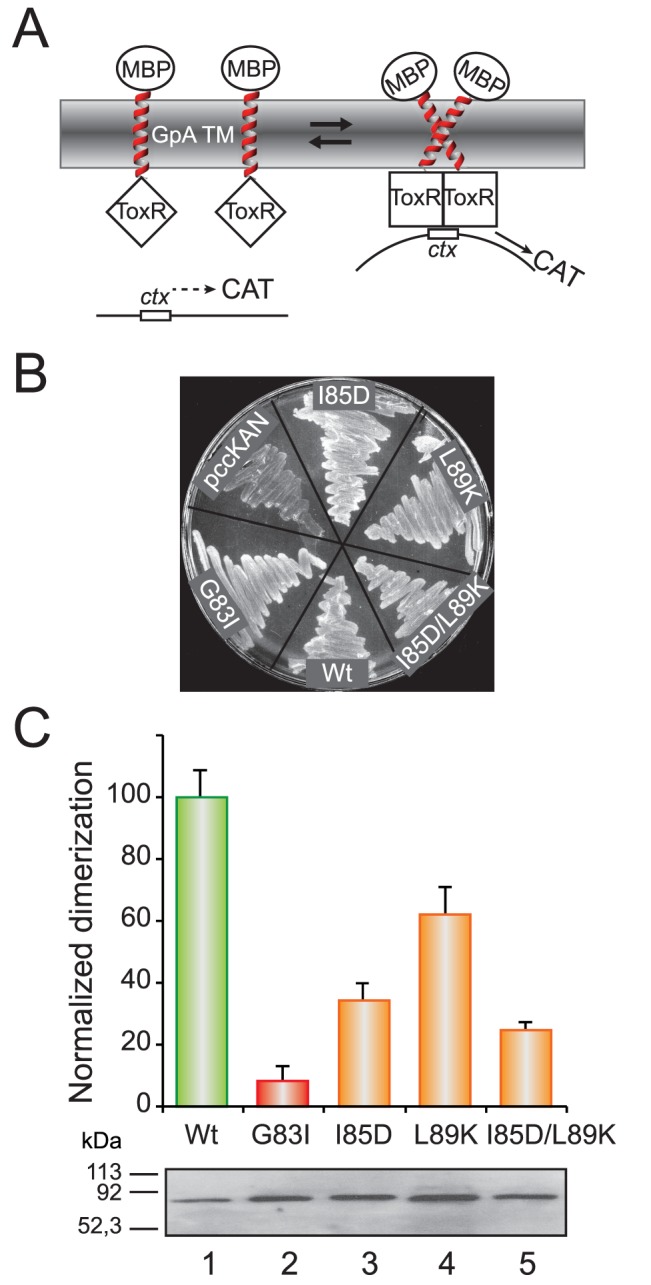
(A) Schematic representation of the ToxCAT assay. ToxR domains (squares) can activate transcription of the reporter gene (CAT) if brought together by the GpA-derived TM domains (right). The maltose binding protein domain (ellipses) helps direct the insertion of the construct into the membrane, complements the malE mutation in the host cells, and serves as an epitope for quantifying the expression level of fusion protein. (B) Complementation assays for wild-type and selected mutant ToxR(GpA)MBP fusion constructs. NT326 cells (malE deficient) carrying various constructs were streaked on a plate with maltose as the sole carbon source and grown for three days at 37°C. All ToxR(GpA)MBP chimeras permit growth of NT326 cells on maltose, while control transformants (pccKAN) do not. (C) Normalized dimerization of the indicated TM domain variants as measured by CAT-ELISA relative to the wild-type GpA TM domain. Bars for intermediate dimerization and non-dimerizing levels are colored orange and red, respectively. Average ± s.d. of results from four independent experiments are shown. Levels of expression of selected ToxR(GpA)MBP constructs as analyzed by immunoblotting are shown at the bottom.
