Figure 3.
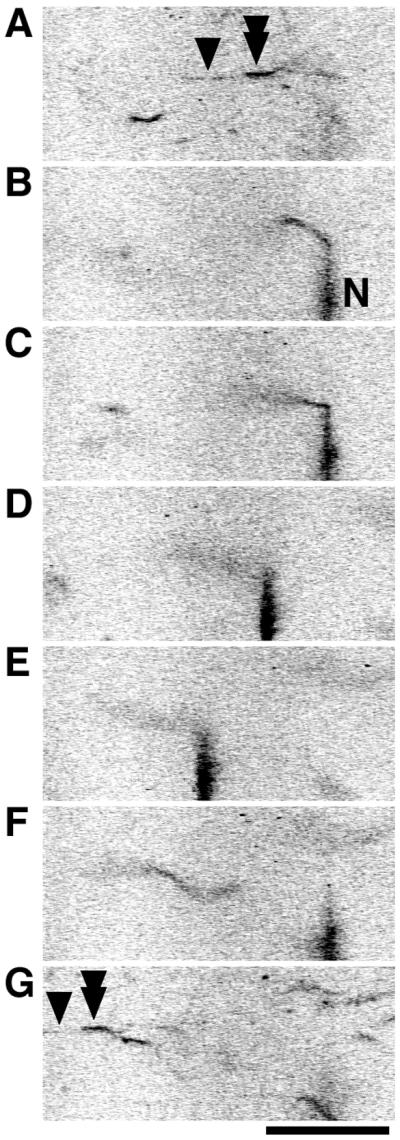
Measurement of binding force of the catch complex. One end of an F-actin filament (tandem arrowhead) was brought into contact with a thick filament (arrowhead) to form the catch complex (A), while the other end was captured with a needle coated with N-ethylmaleimide-treated myosin (indicated by “N”; stiffness of this needle = 18 pN/μm) (B). Then, the microscope stage was moved at a constant velocity of ≈1 μm/s to apply force on the catch complex by the deflection of the needle (C and D). When the deflection of the needle reached a critical extent (21 μm; E), the F-actin filament abruptly detached from the needle. The needle then moved back to the baseline under its own elasticity (F). Even at the break of the bond between the F-actin and the needle, the other end of the F-actin filament (tandem arrowhead) still remained attached to the thick filament (arrowhead) on the glass surface (G). Therefore, the maximal deflection of the needle indicated the lower limit of the binding force sustained by the overlap of the F-actin and thick filaments (1.7 μm). The lower limit of the binding force per overlap was calculated to be 220 pN/μm. (Bar = 20 μm.)
