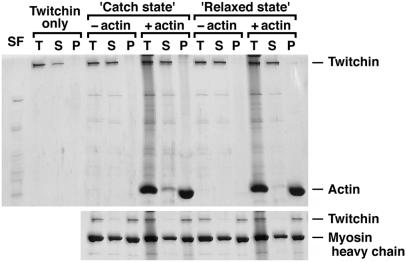
Figure 5.
Cosedimentation of twitchin with and without F-actin either in the absence (Upper) or presence (Lower) of myosin. SF, the soluble fraction present in the experiments with the catch state and the relaxed state; T, total proteins before centrifugation; S and P, the supernatant and precipitate, respectively, after centrifugation sufficient to precipitate 250S molecules. When purified twitchin (4 μg/ml) was centrifuged (Twitchin only), it remained in the supernatant. In the absence of phalloidin-stabilized F-actin (−actin) and of myosin, most of the twitchin remained in the supernatant in both the catch and relaxed states. In the presence of 0.1 mg/ml F-actin (+actin), a little twitchin precipitated in both states with no significant difference in the amounts. In contrast, in the presence of 50 μg/ml myosin (Lower), most of the twitchin precipitated in all conditions. Note that significant amounts of myosin remained in the supernatant in all conditions.