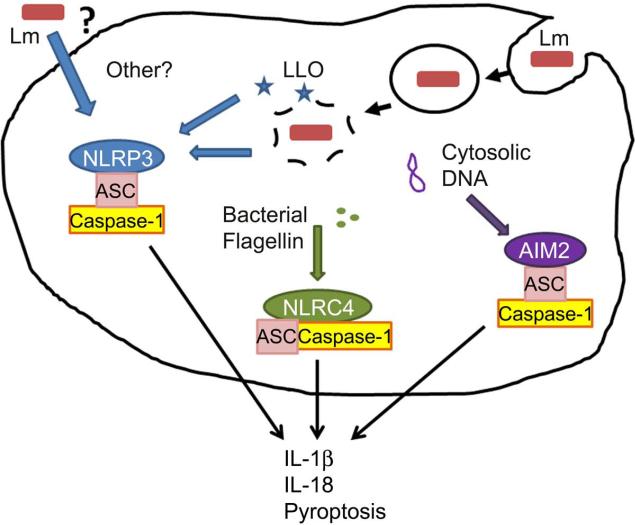
Figure 3. Inflammasome activation by Lm.
In cultured macrophages the activity of the secreted Lm hemolysin (Hly/LLO) is essential for the ability of Lm to escape from phagosomes and growth in the cytosol of infected cells. Mutant ΔHly Lm strains elicit weak IL-1β/IL18 secretion from these cells [103-106], suggesting poor stimulation of cytosolic adaptors and/or poor growth. AIM2 was recently identified as a DNA-sensing inflammasome sensor and mediates secretion of IL-1β and IL-18 by Lm infected macrophages [105,106]. DNA presumably leaks from Lm at a low frequency during infection of the host cell cytosol. Lm flagellin is detected by the NLRC4/Ipaf inflammasome [104]. However, NLRC4 plays very little role in responding to infection by the EGD Lm strain, which suppresses flagellin expression at 37C [106]. Lm strains engineered to over-express Lm or Legionella flagellin hyperactivate the NLRC4 inflammasome [30,107]. Activation of AIM2 and NLRC4 inflammasomes in Lm-infected macrophages also induces cell death via a process termed “pyroptosis” [30,105,107,108]. In these studies, increased pyroptosis in cultured macrophages correlated with reduced bacterial burdens in vivo, suggesting that lysis of infected cells impairs Lm growth or dissemination during systemic infection. The NLRP3 inflammasome is sensitive to a variety of signals, including membrane damage and signaling from dectins or other cell surface receptors [109]. Hly may itself modulate activation of the NLRP3 inflammasome [110], though the mechanism is unclear. The Lm p60 protein also affects secretion of IL-18 [21], suggesting it may activate one or more inflammasomes.