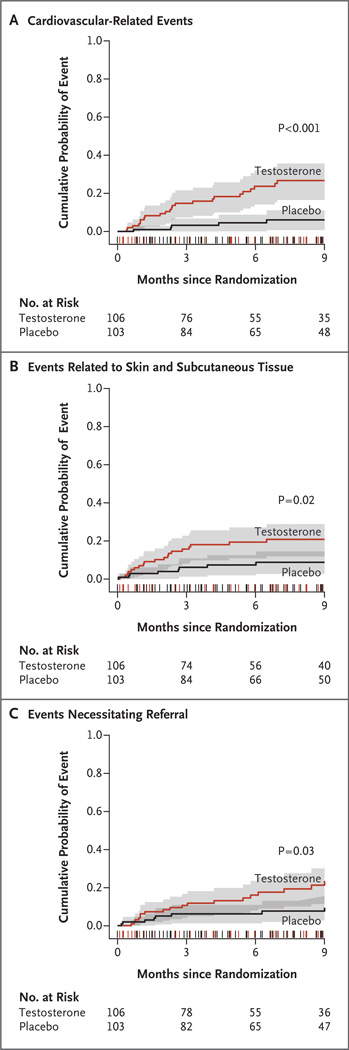
Figure 1. Time-to-Event Analysis of Adverse Events, According to Body System.
Kaplan–Meier estimates of the cumulative probability of incident cardiovascular-related adverse events (Panel A), events related to skin and subcutaneous tissue (Panel B), and events necessitating referral for medical evaluation (Panel C), from randomization to the end of the planned observation phase (9 months after randomization) are shown for the testosterone and placebo groups. The 95% confidence intervals are indicated by the shaded areas. The notches on the x axis show the distribution of censoring times before 9 months among participants in both groups. The P values were calculated from an unadjusted comparison of curves with the use of the log-rank test.