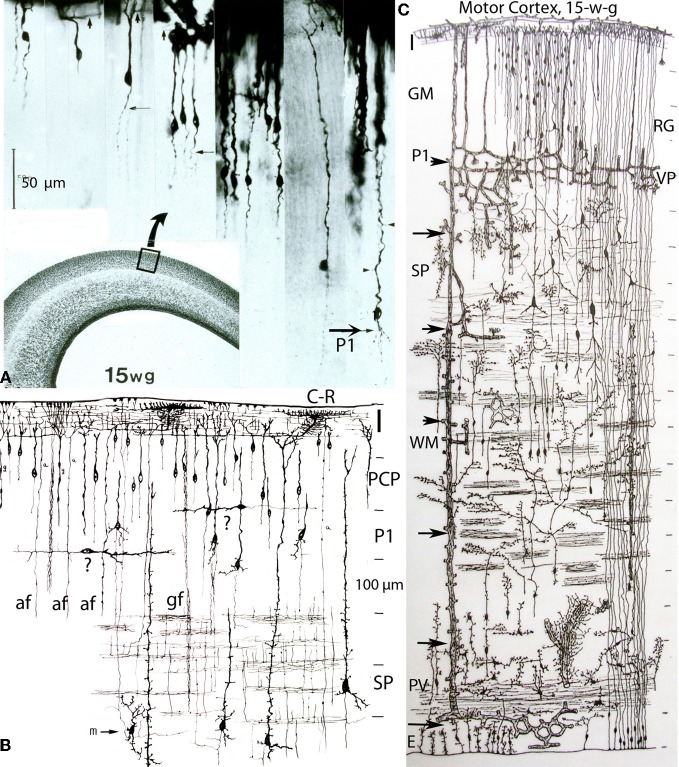
Figure 4.
Composite of a photomicrograph and two camera lucida drawings, from rapid Golgi preparations, illustrating various aspects of the developing cerebral cortex (motor region) of a 15-week-old human embryo. At this age, the thickness of the cerebral cortex, at the motor region, is around 2 mm. (A) Montage of photomicrographs (50 μm scale) showing, at least 7 different pyramidal cell strata with neurons raging in size from 40 μm for the upper, smaller, and last to reach the Cajal-Retzius cells of the first lamina, to 275 μm for the lower, larger, and first to reach the Cajal-Retzius cells. At this age, the deepest and older pyramidal neurons (P1) have started to develop short basal dendrites (arrow) and a few apical dendritic spines (small arrow heads) indicating the beginning of their ascending functional maturation. The pyramidal neurons of the upper strata are still immature with smooth and spineless apical dendrites and descending axon that start to reach the white matter. Cajal-Retzius horizontal axonic fibers (small arrows) are also recognized within the first lamina. The formation of the pyramidal cell plate that started around the 8th week of gestation is nearly complete at this age. (B) Composite of camera lucida drawings (100 μm scale) from rapid Golgi preparations comparing the size, location, interrelations, and organization of the neuronal, and fibrillar elements of the first lamina (I), the pyramidal cell plate (PCP) and the subplate zone (SP). Only the deepest and older pyramidal neurons have started their ascending functional maturation (P1) by developing basal dendrites and their descending axons have entered the white matter. At this age, horizontally migrating neurons (labeled “?”) are first recognized through the cortex lower pyramidal cell strata; we now know these migrating cells are the precursors of the cortex inhibitory neurons. These neurons become incorporated into the deepest, older and maturing pyramidal cell (P1) stratum and will be its future inhibitory (basket, bi-tufted, and chandelier cells) neurons. Also at this age, the subplate zone (SP) deep primordial neurons (pyramidal-like and Martinotti cells) start to lose their original attachment to the Cajal-Retzius cells of the first lamina. (C) Montage of camera lucida drawings (100 μm scale) reproducing the entire thickness of the human motor cortex (at this gestational age), illustrating the size, morphology, distribution, and organization of its basic neuronal, fibrillar, microvascular, and glial elements. Intrinsic capillary anastomotic plexuses, between adjacent perforators, are recognized through the ependymal (E), paraventricular (PV), white matter (WM), subplate (SP), and lower gray matter (GM) zones (arrows). Some perforating vessels reach the paraventricular zone, few reach the white matter and many more reach the gray matter. Fibrous (white matter) and early protoplasmic (gray matter) astrocytes are recognized around their capillaries. At this age, the cortex gray matter starts to develop its first intrinsic microvascularization (VP) through its deepest, older and maturing pyramidal cell (P1) stratum. The remaining gray matter (GM) pyramidal cell strata are still immature at this age. Also, at this age, the deep primordial neurons of the subplate zone start to lose their original functional attachment to first lamina Cajal-Retzius cells. The white matter is crossed by bundles of corticipetal and corticofugal axonic fibers and by numerous ascending glial and neuronal precursors. Glial cell precursors of fibrous astrocytes and oligodendrocytes are recognized accompanying the white matter fibers in both directions. Also illustrated are radial glial cells (RG) attached to the ependyma, with ascending filaments that reach the cortex surface with endfeet incorporated into the EGLM as well as small glial cells precursors still attached to ependyma.