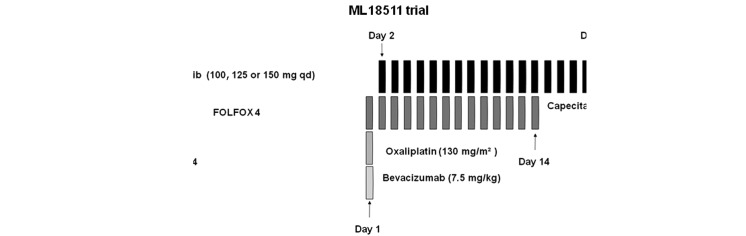
Figure 1.
MO17974 trial: erlotinib, at escalating doses, administered orally from day 3 to day 10, was combined with FOLFOX4 administered every 14 days. ML18511 trial: erlotinib, at escalating doses, was administered on days 2–18 of each 21-day cycle combined with XELOX plus bevacizumab. qd, once a day; bid, twice a day.