Abstract
An urgent need exists for HIV-1 microbicides. Here, we describe the in vivo testing of lactic acid bacteria bioengineered to secrete cyanovirin-N. We fed pigtail macaques a yogurt formulation that used bioengineered strains as a starter culture. Cyanovirin-N expression could be detected in the rectal vault during and immediately after feeding. Ex vivo viral challenge of rectal tissue biopsies revealed that peak viral burden was significantly lower in tissue obtained from experimental animals compared to control animals. Formulation of candidate compounds in lactic acid bacteria and their oral administration appears to be a feasible strategy for mucosal delivery of microbicides.
Keywords: HIV-1, cyanovirin-N, C V-N, lactic acid bacteria, microbicide, pigtail macaque
Introduction
While the world awaits the creation of an effective, prophylactic HIV-1 vaccine, considerable effort has been expended on devising pharmacologic approaches to prevent mucosal HIV-1 transmission. A wide range of microbicidal drugs that act at extra- and intracellular stages of the HIV-1 life cycle have been tested in animal models of viral transmission [1, 2]. Mucosal application of antiretroviral drugs (ARV) such as reverse transcriptase inhibitors prevents vaginal and rectal HIV-1 transmission in macaque and humanized mice models of purposeful infection [3, 4]. The latter approach has been clinically translated and encouraging results from recently completed trials appear to validate ARVs as potential first line microbicides when administered vaginally or as oral preventatives [5, 6]. Obvious limitations of using ARVs derive from their cost, side effect profile and potential loss of efficacy against drug resistant viruses.
Besides ARVs, the microbicide pipeline includes numerous other compounds that are efficacious in animal models including a number of protein molecules such as neutralizing antibodies and fusion inhibitors [7]. An additional virucide is the compound cyanovirin-N (CV-N), an 11-kDa protein originally isolated from Nostoc ellipsosporum (green blue algae) [8, 9]. CV-N harbors anti-HIV activity at low nanomolar range by selectively binding to high mannose residues in the viral envelope thereby blocking cellular entry [10]. Positive attributes of CV-N include its broad activity against human and primate immunodeficiency viruses and lack of cytotoxicity even at high concentrations. Additionally, direct rectal application of CV-N in 1% or 2% gel formulation prevents mucosal transmission of Simian HIV in macaque models [11, 12].
Protein compounds with anti-HIV-1 activity may be extremely potent and nontoxic, target a diverse swarm of viruses, and retain sufficient potency to thwart the emergence of resistance, yet their clinical utility remains questionable. The prohibitive cost of their manufacture and generally labile profile in the presence of numerous proteases argue against their further development as commercially viable drugs. Their formulation poses additional challenges and currently relies upon direct mucosal instillation. Furthermore, as with all microbicides, their dosing schedules need to assure adequate mucosal levels of drug during at-risk activity requiring high levels of adherence. Here, we describe a potential strategy to overcome many of these hurdles by using an alternative approach for mucosal delivery of protein microbicides. We and others have previously described the ease with which lactic acid bacteria (LAB) can be genetically manipulated to secrete proteins with anti-HIV-1 activity [13-16]. Here, we used LAB that had been bioengineered to secrete CV-N (LAB-CV-N). We formulated LAB-CV-N as a yogurt product and fed pigtail macaques 50 ml daily. We could detect CV-N in the rectal vault, rectal lavage and stool during feeding. To evaluate antiviral activity, rectal biopsies obtained immediately before and after LAB-CV-N administration underwent ex vivo viral challenge. LAB-CV-N administration was associated with 20-fold lower levels of peak viral replication in tissue culture (n=4; p=0.025). Furthermore CV-N expression could be detected in rectal lavage samples up to 7 days after cessation of feeding. All animals tolerated treatment and no animals were colonized by recombinant bacteria. Formulation of protein virucides in LAB based food vehicles appears to be a promising strategy for their mucosal delivery.
Methods
Bioengineering LAB for secretion of CV-N
Numerous strains of LAB are known to exist as commensal organisms in humans and we initially chose strains for genetic engineering that were known inhabitants of the gastrointestinal and/or vaginal microbiome. Strains were compared for their ability to secrete CV-N. A previously described expression system was used to create LAB-CV-N that involved the insertion of a codon optimized CV-N gene in a plasmid construct with appropriate LAB-specific signals for robust recombinant protein production and secretion [13]. The expression vector was electroporated into LAB by using Gene Pulser II Electroporation System (Bio-Rad Laboratories, Hercules, CA).
CV-N detection
Western blot was used to detect CV-N secretion using previously described methods [13]. The sensitivity of CV-N western blot was 0.1 ng, as determined by experiments using known input of recombinant CV-N (kind gift of James B. McMahon and Michael R. Boyd). Protein extracts from LAB-CV-N supernatants were run on NuPAGE® 4-12% Bis-Tris precast Gel (Invitrogen) by using XCell SureLock® Mini-Cell (Invitrogen). After electrophoresis, the resolved proteins were transferred onto PVDF membrane by using XCell II ® Blot Module. Upon the completion of transfer, standard protocols of WesternBreeze® Chemiluminescent Detection Kits (Invitrogen) were followed and developed on ECL Hyperfilm (GE Healthcare, Piscataway, NJ) according to manufacturer’s instructions. Image acquisition of western blot was performed through Epson scan (Version 2.68a) by using an Epson Perfection 4990 photo scanner operating at film mode with the following settings: 256 gray shades and a resolution of 300 dpi. To quantify bands, Quantity One® (Bio-Rad Laboratories) basic (version 4.62) was used to measure densitometry.
CV-N quantification
The Protein Detector HRP Microwell Kit from KPL (Gaithersburg, MD) was used to build an in-house CV-N ELISA. Briefly, lavage samples were concentrated by employing Amicon Ultra-0.5 Centrifugal Filter Unit with Ultracel-3 membrane (Millipore, Billerica, MA) and combined with coating solution, pipetted onto Nunc MaxiSorp® plates and incubated overnight. After blocking with 1% BSA, the plates were incubated with an anti-CV-N primary antibody (kind gift of Drs. Boyd and McMahon) for one hour prior to adding a secondary HRP anti-rabbit antibody for an additional hour incubation. Plates were washed thrice after each antibody treatment and substrate (ABTS®) solution was added and developed for 30 minutes. A SpectraMax® M5 (Molecular Devices, Sunnyvale, CA) plate reader was used to measure light absorbance at 405nm. The sensitivity of CV-N ELISA was 1.0 ng, as determined by experiments using known input of recombinant CV-N.
Protein extraction from stool
One gram of unconcentrated stool was suspended in 1 ml of a fecal extraction buffer, which consisted of Tris buffer isotonic saline (150 mmol/L), with 10 mmol/L CaCl2 and 0.25 mmol/L thiomersal as an antimicrobial agent (pH 8.4) [17] and homogenized for 3 minutes followed by centrifugation. The top halves of the supernatants then were pipetted off, frozen, and stored at −20°C until assay.
CV-N formulation
Animal studies, described below, used LAB-CV-N that was formulated as yogurt. Briefly, 109 Colony Forming Units (CFU) of LAB-CV-N were admixed with scalded 500 ml non-fat milk and incubated over night at 37° C, as described previously [18]. Product was stored in daily 50 ml doses and frozen.
Animal Studies
Four sexually mature, healthy female pigtail macaques (average age = 9.5; range = 7-14 years) were used in an IACUC approved protocol. In order to reliably dose study animals with known quantities of the test yogurt, each animal was trained to accept refrigerated yogurt from a feeding syringe and (more successfully) to eat frozen yogurt in 10 ml and 20 ml cubes. All experimental feedings were observed by research personnel. The study procedures, as noted below, were performed after animals were sedated and placed in a prone position with knees tucked under their abdomen. Several feeding trials were completed and animals received 4 to 14 days of yogurt. Rectal lavage and swabbing was performed at baseline, during feeding and up to 7 days after cessation of feeding in 3-4 day intervals. Swabs were immediately placed in MRS media and underwent culture for 48 hrs. Bacterial supernatant was assayed for CV-N production using western blot. For rectal lavage, approximately 2 ml of sterile saline was instilled in the rectal vault then collected, divided into two equal aliquots and immediately snap frozen. Rectal biopsies were obtained at baseline and 24 hours after 3 days of LAB-CV-N administration. Briefly, forceps were used to lift rectal epithelium and three 2-3 mm biopsies of epithelial mucosa were collected and underwent several washes with antibiotic cocktail (100 units/ml penicillin, streptomycin, nystatin, amphotericin B) and PBS. The biopsies were mailed overnight on cold packs from the University of Washington, Seattle to our laboratory where they were immediately layered onto 48 well plates on surgifoam (Johnson & Johnson) in RPMI 1640 containing IL-2, penicillin/streptomycin and 20% FCS. Tissue sections were challenged with 250 ul (TCID 50) of RT-SHIV (SIVmac239 with the Reverse Transcriptase replaced with that of HIV-1 clone HXBc2. NIH AIDS Research and Reference Reagent Program catalog #11342) and maintained over 20 days. Supernatant was collected in 3-4 day intervals and tested for infectivity using the TZM-bl indicator cell line.
Statistical analysis
We quantified levels of viral replication by averaging luciferase activity associated with each rectal tissue biopsy following ex vivo viral challenge. The biopsies were obtained before and after LAB-CV-N administration or after sham-yogurt administration. For each biopsy, levels of viral replication were quantified at ~3 day intervals over 20 days and normalized to allow for inter-comparisons. We incorporated both the repeated experiments within an animal as well as the paired nature of the study for which each animal contributed equally to the control and treatment arms of the experiment. The descriptive measure for each run of the experiment was a slope which measured the change in viral replication over time. The average slope of each animal as a control was compared to its overall average from the tissues contributed after being treated. The two-sided t-test was conducted to identify if the difference in slopes across all animals was significantly different from zero (i.e., different from no effect due to treatment). Similar methods were used to compare the treatment difference in the maximum value of viral replication for each animal.
Results
Formulation of LAB-CV-N
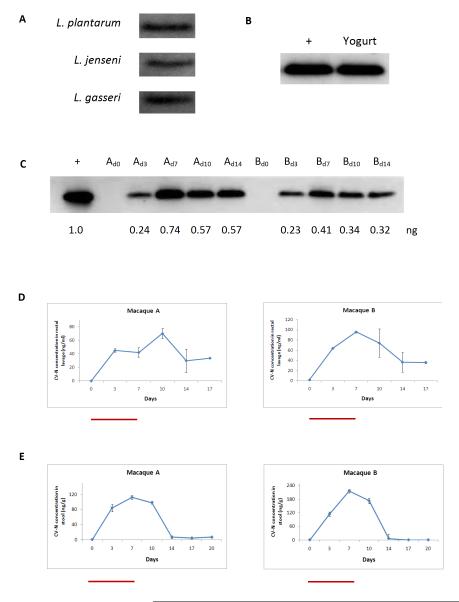
Fermented food products have remained a staple of the human diet for millennia. The use of LAB as components of starter cultures is used widely in the dairy industry and recent advances in the genetic modification of LAB have allowed investigators to use engineered strains as drug delivery vehicles for a range of bioactive compounds including cytokines and viral antigens. Prior to commencing animal studies, we first compared the efficiency of bioengineering CV-N in three species of LAB known to be commensal organisms in the human gut (L. plantarum) and vagina (L. jensenii, L. gasseri). As seen in Figure 1a, all three species supported CV-N secretion at roughly equivalent levels. We chose L. plantarum for all downstream studies given our focus on engineering CV-N production in the rectal vault. We first verified that the yogurt fermentation process preserved the biosynthetic capacity of LAB-CV-N. Approximately 100 μl of product was spread on plates and incubated at 37° C overnight. The resulting bacterial clones were picked and independent clonal cultures were started. During log growth phase (24-48 hours), supernatants were harvested and as seen in Figure 1b, western blot revealed CV-N production in supernatants.
Figure 1.
A. Comparison of the ability of various strains of LAB to support CV-N production and secretion. Strains were transformed and western blot detection of CV-N in 10 μl of bacterial supernatant revealed roughly similar biosynthetic potential in L. plantarum, L. jensenii, and L. gasseri.
B. Standard fermentation procedures were used to create a yogurt product with LAB-CV-N as starter culture. Secondary culture of yogurt led to the identification of CV-N in culture supernatant. A known amount (1 ng) of recombinant CV-N isolated from bacterial supernatant was used as a positive control in all western blots as denoted in ‘+’ lane.
C. Western blot of supernatants of secondary culture of rectal swab samples from two macaques (A, B) during and immediately after 14 days of LAB-CV-N feeding. CV-N could be detected during all days of feeding. Samples were collected on days 0, 3, 7, 10 and 14 for macaques A and B and 5 ul was loaded onto each well. A known amount (1 ng) of recombinant CV-N isolated from bacterial supernatant was used as a positive control in all western blots as denoted in ‘+’ lane. Values under each band correspond to CV-N quantification (ng) as calculated by Quantity One® software.
D. CV-N levels were quantified by ELISA in rectal lavage samples during 7 days of LAB-CV-N feeding and afterwards (days 8-17). Red bars denote days of feeding. Peak levels were achieved at 70 ng/ml (macaque A) and 100 ng/ml (macaque B) on days 10 and 7, respectively. Importantly, CV-N could still be detected in rectal lavage for up to ten days after cessation of feeding in both animals.
E. Post-feeding levels of CV-N were detected in stool in two animals. Levels are denoted as ng CV-N per gram of unconcentrated stool. CV-N could be recovered from stool with peak levels of ~120-220 ng/g on day 7.
In vivo retention kinetics and biosynthetic activity of LAB-CV-N
To better understand the in vivo dynamics of LAB-CV-N, we first fed 2 animals with product over a 14 day period. Animals were subjected to rectal swabbing at baseline and at 3-4 day intervals over 20 days. As seen in Figure 1c, culture of rectal swabs led to CV-N detection in bacterial supernatants during all 14 days of feeding starting at the first measurement at day 3. This successful trial run prompted us to shorten the duration of feeding to 7 days and to measure CV-N levels in rectal lavage and stool. As seen in Figure 1d, CV-N could be detected in rectal lavage during and up to 10 days after the cessation of feeding with peak levels of ~70-100 ng/ml on days 7-10. CV-N could also be recovered from stool with peak levels of ~120-220 ng/g on day 7 but fell to levels below detection by day 14 (Figure 1e).
Antiviral efficacy of LAB-CV-N
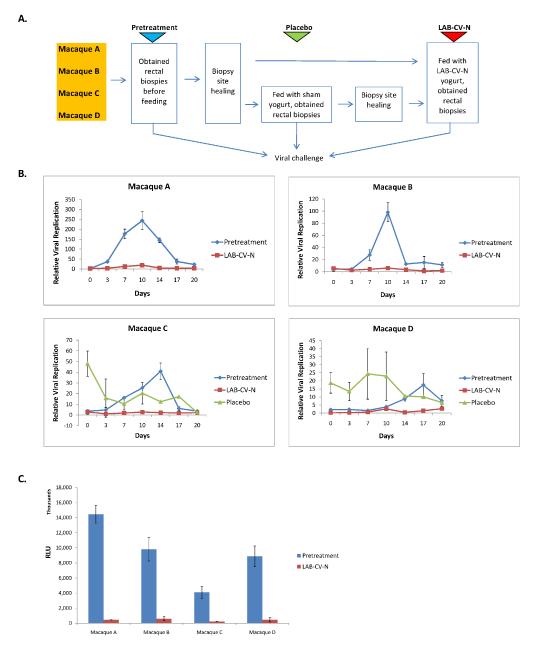
The ultimate success of a HIV-1 microbicidal drug is dependent upon its ability to prevent the initial round of viral replication in mucosal tissue. To determine the effect of LAB-CV-N in preventing viral replication in rectal tissue, we conducted trials in which we fed animals over a three day period either yogurt made from wild-type L.plantarum (n=2) or L.plantarum-CV-N (n=4). All animals underwent a pre-treatment biopsy 2 months prior to yogurt administration. Upon yogurt administration, rectal biopsies were obtained on day 4, placed in culture and challenged with virus. The overall experimental schema is shown in Figure 2A. As seen in Figures 2B and C, quantification of levels of ex vivo viral replication in tissue obtained from four animals over a 20 day period revealed up to 20-fold lower levels of maximal viral replication in LAB-CV-N treated animals compared to tissues obtained before LAB-CV-N treatment (paired t-test p-value=0.025). When we analyzed all data over the 20 day period, the treatment samples were found to have lower rate of change in viral replication than the control samples (i.e., −0.0167) but this difference was not statistically significant (paired t-test p-value=0.142).
Figure 2.
A. Schematic of yogurt feeding regimen and biopsy timing in four macaques.
B. Levels of ex vivo viral replication in rectal tissue isolated from animals that had received either no treatment, LAB-CV-N or LAB-placebo. Tissue sections were kept in culture over 20 days and levels of RT-SHIV replication were quantified by a TZM-b1 reporter assay. Maximum levels of viral replication were up to 20-fold lower in LAB-CV-N treated animals compared to animals that had not received any treatment (p=0.025).
C. Inter-comparison of peak values of RT-SHIV replication in rectal tissues from four macaques before and after treatment with LAB-CV-N. Data are presented as mean levels of luminescence (RLU) +/- SD.
Discussion
The recent CAPRISA004 trial has conclusively demonstrated the preventive potential of HIV-1 microbicides [5]. The data above suggests that LAB can be further developed as HIV-1 preventatives and may overcome two major hurdles for the commercial and clinical development of protein microbicides: the cost of manufacture and the relatively short half-life upon direct mucosal instillation. Our experiments involved CV-N and demonstrated delivery to the rectal vault after oral intake of bioengineered yogurt-an approach that may be especially relevant to decreasing HIV-1 transmission in men who have sex with men (MSM), a demographic sector with continued high rates of virus transmission. While peak levels of viral burden were significantly lower in CV-N treated animals, the product did not completely eliminate viral replication suggesting that a multi-modal approach may be required to improve antiviral potency. For example, it may be possible to engineer the secretion of numerous antiviral molecules in a single LAB or introduce a combination of LAB, each engineered to secrete a chosen antiviral molecule. While ours was a pilot study to determine LAB-CV-N uptake and retention kinetics in vivo, the next steps in testing antiviral efficacy of LAB based reagents must include in vivo cross comparisons to leading microbicide candidates (e.g. tenofovir). Although the number of animals studied is small, it is interesting to note that the protective effect of CV-N was more durable than expected. For example, despite rigorous washing of primary rectal biopsy material, we still observed lower levels of peak viral replication in tissues obtained from LAB-CV-N treated animals over a 20 day ex vivo culture period. One explanation could be CV-N binding to macaque rectal epithelial cells and mediating protection even after cessation of feeding. This conjecture is further supported by the detection of CV-N in lavage samples but not in stool 10 days after cessation of feeding. Perhaps the mechanical forces of lavage dislodged residual CV-N from epithelia allowing its detection in lavage even after the complete elimination of the source recombinant bacteria. Alternatively, the discrepancy in CV-N detection in stool vs lavage may simply be due to stool substrates not being concentrated prior to analysis and thus containing CV-N below limits of detection by ELISA and/or western blot. Our proof-of-principle experiments involved an LAB expression system that utilized an antibiotic selection gene thereby accounting for rapid elimination of the bioengineered strains in vivo. Recent advances in the genetic manipulation of LAB allow insertion of chosen protein encoding sequences directly into the bacterial genome thereby avoiding the use of drug resistance markers [19,20]. Such approaches could be used to generate clinical grade reagents as is being done in several European centers. Lastly, the success of any potential microbicide is dependent upon several factors including cost, ease of use and duration of effect. LAB based products would appear to satisfy each of these requirements. More work to increase overall potency clearly needs to be performed to achieve sterilizing levels of activity in mucosal surfaces.
Acknowledgements
This work was supported by NIH U19AI065430, NIH P30AI042853 and NIHP20RR025179. M. Li was supported by NIH T32DA013911. We thank James B. McMahon (NCI) and Michael R. Boyd (USA Mitchell Cancer Institute, Mobile, AL) for provision of recombinant CV-N and anti-CV-N antibody.
Source of funding: NIH U19AI065430, NIH P30AI042853 and NIHP20RR025179
Footnotes
Declaration: Authors have no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veazey RS, Ketas TJ, Dufour J, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. Sep 1;202(5):739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denton PW, Krisko JF, Powell DA, et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One. 5(1):e8829. doi: 10.1371/journal.pone.0008829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005 Nov 3;438(7064):99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 4.Veazey RS, Springer MS, Marx PA, Dufour J, Klasse PJ, Moore JP. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005 Dec;11(12):1293–1294. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 5.Karim Q Abdool, Karim SS Abdool, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veazey RS, Shattock RJ, Pope M, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003 Mar;9(3):343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 8.Boyd MR, Gustafson KR, McMahon JB, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997 Jul;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori T, Boyd MR. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. Antimicrob Agents Chemother. 2001 Mar;45(3):664–672. doi: 10.1128/AAC.45.3.664-672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffa V, Stieh D, Mamhood N, Hu Q, Fletcher P, Shattock RJ. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol. 2009 Jan;90(Pt 1):234–243. doi: 10.1099/vir.0.004358-0. [DOI] [PubMed] [Google Scholar]
- 11.Tsai CC, Emau P, Jiang Y, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003 Jul;19(7):535–541. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CC, Emau P, Jiang Y, et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004 Jan;20(1):11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 13.Pusch O, Boden D, Hannify S, et al. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J Acquir Immune Defic Syndr. 2005 Dec 15;40(5):512–520. doi: 10.1097/01.qai.0000187446.76579.d3. [DOI] [PubMed] [Google Scholar]
- 14.Pusch O, Kalyanaraman R, Tucker LD, Wells JM, Ramratnam B, Boden D. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS. 2006 Oct 3;20(15):1917–1922. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 15.Vangelista L, Secchi M, Liu X, et al. Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrob Agents Chemother. Jul;54(7):2994–3001. doi: 10.1128/AAC.01492-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang TL, Chang CH, Simpson DA, et al. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11672–11677. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattar AF, Coran AG, Teitelbaum DH. MUC-2 mucin production in Hirschsprung’s disease: possible association with enterocolitis development. J Pediatr Surg. 2003 Mar;38(3):417–421. doi: 10.1053/jpsu.2003.50071. discussion 417-421. [DOI] [PubMed] [Google Scholar]
- 18.Venkatachary V. Lifco’s How to Cook. 1st ed The Little Flower Co; Chennai: 1987. [Google Scholar]
- 19.Martin MC, Alonso JC, Suarez JE, Alvarez MA. Generation of food-grade recombinant lactic acid bacterium strains by site-specific recombination. Appl Environ Microbiol. 2000 Jun;66(6):2599–2604. doi: 10.1128/aem.66.6.2599-2604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steidler L, Neirynck S, Huyghebaert N, et al. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003 Jul;21(7):785–789. doi: 10.1038/nbt840. [DOI] [PubMed] [Google Scholar]