Abstract
Purpose.
Lacritin is a human tear glycoprotein that promotes basal tear protein secretion in cultured rat lacrimal acinar cells and proliferation of subconfluent human corneal epithelial cells. When topically added to rabbit eyes, lacritin promotes basal tearing. Despite these activities on several species, lacritin's presence in nonprimate tears or other tissues has not been explored. Here we probed for lacritin in normal horse tears.
Methods.
Sequences were collected from the Ensembl genomic alignment of human LACRT gene with high-quality draft horse genome (EquCab2.0) and analyzed. Normal horse tears were collected and assayed by Western blotting, ELISA, and mass spectrometry. Newly generated rabbit antibodies, respectively, against N- and C-terminal regions of human lacritin were employed.
Results.
Identity was 75% and 45%, respectively, at nucleotide and protein levels. Structural features were conserved, including a C-terminal amphipathic α-helix. Anti-C-terminal antibodies strongly detected a ∼13 kDa band in horse tears that was validated by mass spectrometry. In human tears, the same antibody detected uncleaved lacritin (∼24 kDa) strongly and C-terminal fragments of ∼13 and ∼11 kDa weakly. Anti-N-terminal antibodies were slightly reactive with a ∼24 kDa horse antigen and showed no reaction with the anti-C-terminal–reactive ∼13 kDa species. Similar respective levels of horse C-terminal versus N-terminal immunoreactivity were apparent by ELISA.
Conclusions.
Lacritin is present in horse tears, largely as a C-terminal fragment homologous to the mitogenic and bactericidal region in human lacritin, suggesting potential benefit in corneal wound repair.
Protein evidence for the first nonprimate lacritin is described.
Introduction
The physiological significance of individual tear proteins and their complexes is a growing area of investigation. Lacritin, discovered in 2001, is a tear glycoprotein with multiple functions.1 Lacritin is mitogenic for nonconfluent corneal epithelial cells2 and stimulates basal tear secretion by lacrimal acinar cells.1 Topical lacritin promotes basal tearing in rabbit eyes3 and appears to be a secretogogue for tear film mucin MUC16 (Laurie GW, et al. IOVS 2006;47:ARVO E-Abstract 1606). New data reveal that a C-terminal proteolytic fragment is bactericidal against gram negative and positive bacteria (McKown RL, et al. IOVS 2010;51:ARVO E-Abstract 4181). Several small clinical studies suggest that only 4% to 5% of tear proteins are downregulated in dry eye4,5 or blepharitis,6 of which lacritin is the only prosecretory protein apparently affected.7 Lacritin may thus play a key role in the physiology of the ocular surface, in which its deficiency might contribute to ocular disease. Whether or not this is the case will require hundreds of samples, high-throughput assays, and attention to the lacritin cell surface targeting mechanism that includes syndecan-1 and tear heparanase.8
Although genomic alignments suggest the existence of lacritin orthologs in several mammals,5 expression has been documented only in human1 and nonhuman primates.9 Partial genomic alignment of the human LACRT gene with the homologous region in horse chromosome 6 was sufficient to warrant collecting and assaying horse tears by Western blotting and enzyme-linked immunosorbent assay (ELISA). Horses commonly suffer from corneal ulceration, often resulting in hospitalization,10 as well as dry eye. Here we probed for lacritin in normal horse tears toward a more comprehensive understanding of lacritin function in mammals.
Materials and Methods
Genomic and Protein Analyses
Ensembl (release 67; http://uswest.ensembl.org/index.html, in the public domain); genomic alignment of human LACRT with the EquCab2.0 horse genome11 was analyzed, first by BLASTX using human lacritin protein sequence as query. Untranslated sequence was excluded, as guided by AceView analysis of human exons, in keeping with data from lacritin genomic cloning.1 Horse nucleotides in alignment with human exons 1 through 5 were assembled into a single nucleotide coding sequence and then translated using the ExPASy Translate tool. The same process was performed for cat, dog, and chimp. Comparative alignments were performed by ClustralW. Analysis of putative protein structure and modification was by PONDR, PSIPRED, PEPWHEEL, SignalP, and NetOGlyc.
Tear Collection
Tears were collected from normal eyes of three horses by application of an ophthalmic sponge (Aspen Surgical, Caledonia, MI) to the medial canthus of the eye for approximately 20 to 30 seconds. Tear-containing sponges were individually stored in 0.5 mL Eppendorf tubes at −80°C. Collection was conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Virginia Tech Institutional Animal Care and Use Committee. At the time of analysis, sponges were thawed, incubated for 20 minutes in 60 μL PBS, and centrifuged for 10 minutes at 8000g in the same 0.5 mL Eppendorf tubes, whose bottoms were perforated for centrifugation. Each was inserted into a 1.5 mL Eppendorf tube for collection of eluant. Protein concentration was determined using a BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL) with bovine serum albumin as the protein standard. Tear samples were collected from normal human eyes under proparacaine anesthesia at the Walter Reed Army Medical Center using 2 × 10 mm polyester rods (Filtrona, Richmond, VA) as described elsewhere.12 Human tear collection was approved by the Walter Reed Institutional Review Board and was conducted in adherence to the tenets of the Declaration of Helsinki. Rods were stored at −80°C.
SDS-PAGE, Western Blotting
Tear samples were loaded onto 4% to 20% Mini-PROTEAN TGX precast gels (Bio-Rad, Hercules, CA), electrophoresed at 200 V, and either stained with 0.08% Coomassie Brilliant Blue R-250 or transferred to nitrocellulose (Protran BA 83; Whatman, Dassel, Germany). Blots were blocked with PBS-Tween ([PBS-T] PBS with 0.3% Tween-20 [Sigma-Aldrich, St. Louis, MO]), incubated with rabbit anti-N-terminal (1:200)– or anti-C-terminal (1:200)–specific anti-lacritin antibodies12 for 2 hours at room temperature, rinsed with PBS-T, and incubated for 2 hours at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (MP Biomedicals, Solon, OH) diluted 1:10,000 in PBS-T. Blots were washed with PBS-T and developed via chemiluminescence with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc., Rockford, IL).
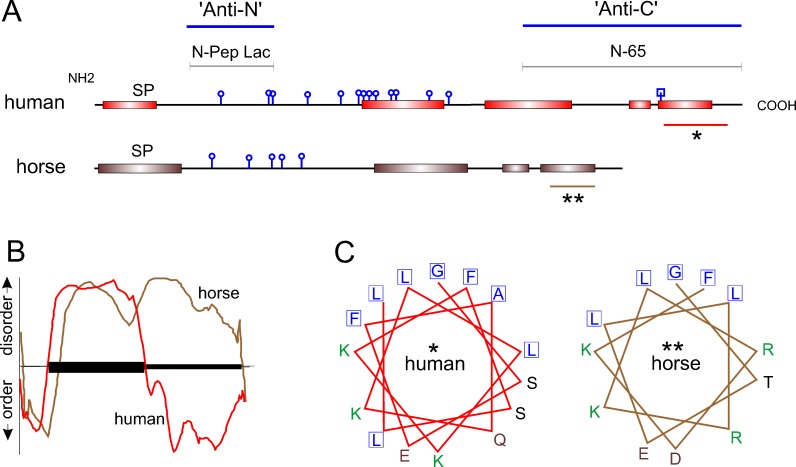
The anti-N-terminal antibody is directed against synthetic peptide N-Pep Lac corresponding to human lacritin amino acids 20 through 38 (EDASSDSTGADPAQEAGTS; Seifert et al.12 The anti-C-terminal antibody is directed against human lacritin truncation mutant N-65.12 The location of both antigens is indicated in Figure 2.
Figure 2. .
Comparative analysis of predicted secondary structure. (A) NetOGlyc 3.1 predicts at least five O-linked glycosylation sites (round knobs) in horse versus 13 in humans. N-linked glycosylation centered on asparagine 119 in human lacritin is predicted by NetNGlyc 1.0 (square knob). PSIPRED (version 3.0) predicts several C-terminal α-helices in horse lacritin, much like human. The two large C-terminal helices in human lacritin have been validated by circular dichroism2 (Beck SL, Laurie GW, unpublished data, 2005). The human N-Pep Lac peptide and recombinant truncation mutant N-65 are antigens for anti-N- and anti-C-terminal lacritin antibodies noted in Figure 1. SP, signal peptide. Asterisks indicate regions further analyzed. In human lacritin, the C-terminal α-helix (*) forms the syndecan-1 binding domain for cell targeting.8 (B) PONDR analysis of human and horse lacritin sequences. The y-axis indicates predicted disorder and order, respectively, above and below the x-axis. Protein length is represented along the x-axis with the amino terminal signal peptide indicated by the ordered domain at left. The slightly longer human lacritin tracing has been compressed to fit on the same x-axis scale with the horse. PONDR predicts that human lacritin may contain more C-terminal order than horse lacritin. (C) PEPWHEEL analysis of α-helical regions indicated by asterisks in (A). The distinctive hydrophobic face (adjacent boxed residues) of this region in human lacritin2 is partially conserved in horse lacritin. The human hydrophobic face is required for syndecan-1 binding, a function predicted to be shared by horse lacritin. Amino acids with nonpolar, basic, acidic, and uncharged side chains are respectively blue, green, brown, and black.
Mass Spectrometry
Mass spectrometric analysis was performed on the 13 kDa band excised from a 1-D SDS-PAGE gel of horse tears. Gel protein was extracted in 5× Invitrosol LC/MS Protein Solubilizer (Life Technologies, Carlsbad, CA), diluted in 100 mM ammonium bicarbonate to 1× Invitrosol, and reduced in dithiothreitol (DTT) at 60°C for 30 minutes; then reduced cysteine residues were blocked with iodoacetamide for 15 minutes at room temperature in the dark. Extracted protein was trypsin digested at an enzyme-to-substrate ratio of 1:20 (wt/vol) for 2 hours at 37°C before quenching by acidification. Capillary liquid chromatography tandem mass spectrometry (Cap-LC/MS/MS) was performed on a Thermo Finnigan LTQ mass spectrometer (Thermo Scientific, West Palm Beach, FL) equipped with a CaptiveSpray Ionization source (Bruker-Michrom, Auburn, CA), operated in positive ion mode, and coupled to an UltiMate 3000 LC System (Dionex, Sunnyvale, CA). Peptides were first separated on a Magic C18 column (0.2 × 150 mm; Bruker-Michrom) and eluted into the LTQ system over a run time of 65 minutes. MS/MS was acquired according to standard conditions. Briefly, analysis was programmed for a full scan recorded between 0.35 and 2 kDa and a MS/MS scan to generate product ion spectra for amino acid determination. Sequence information from the MS/MS data was processed by converting .raw files into a merged file (.mgf) that was then searched using MassMatrix13 for tandem mass spectrometric analysis. Protein/peptide identifications were checked manually for validation.
ELISA
Plates were coated overnight at 4°C with 100 μL horse tears diluted to 10 μg total protein/mL with coating buffer (0.017 M NaHCO3, 0.015 M Na2CO314), or with human recombinant lacritin generated from plasmid pLAC as previously described.14 Lacritin was diluted in the same coating buffer to generate a standard curve of 0, 0.5, 1, 2, 4, 6, and 8 ng.12 Wells were washed and blocked with PBS-T and then incubated for 1 hour at 37°C with anti-N- or -C-terminal anti-lacritin antibody (1:200 in PBS-T), washed with PBS-T, incubated for 1 hour at 37°C with goat anti-rabbit IgG (1:1000; MP Biomedicals, Solon, OH), washed with PBS-T, and finally treated with o-phenylenediamine (OPD) substrate (Acros Organics, Geel, Belgium) for 10 minutes, followed by measurement at OD 415 in a Bio-Rad 680 ELISA plate reader.
Statistical Analysis
Values are expressed as the mean ± SD. Paired t-tests were performed using Prism (GraphPad Software, La Jolla, CA). Molecular weight analysis was performed using ImageJ (http://rsweb.nih.gov/ij/, in the public doman).
Results
Genomic Alignment
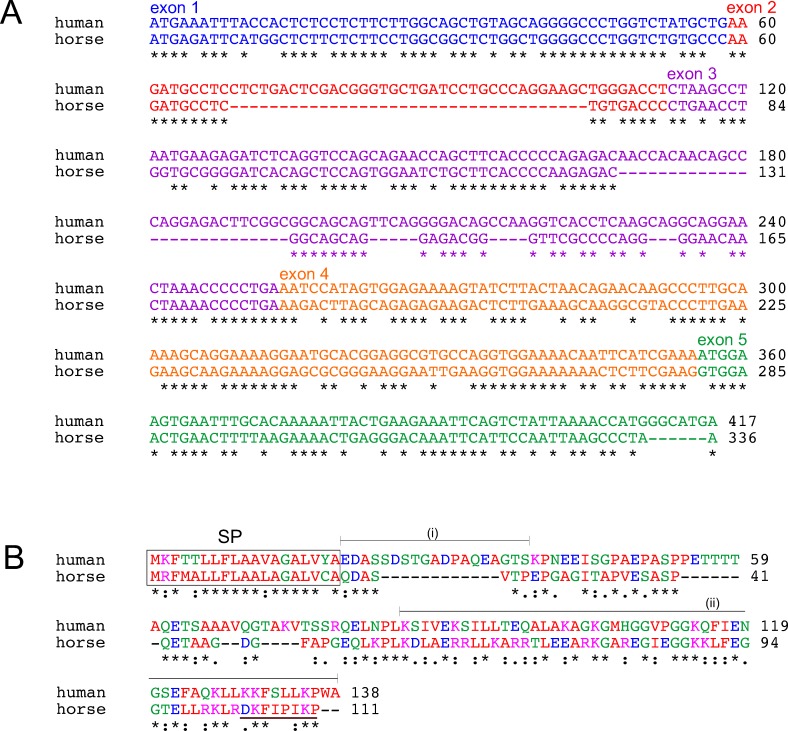
Ensembl aligned the human LACRT gene (chromosome 12; nucleotides 44,024,595–55,028,679) with horse chromosome 6 (nucleotides 71,395,011–71,398,029; http://useast.ensembl.org/, in the public domain). We excluded expected untranslated sequence as per alignment with Ensembl-designated exons and AceView, and then extracted and assembled homologous horse exons into a single coding sequence for comparison to human by ClustralW. Alignment revealed 75% identity overall (Fig. 1A and Table). Similar ClustralW comparison of human with confirmed chimp (Pan troglodytes) and cat (Felis catus) lacritin orthologs revealed respective nucleotide identities of 99% and 72%. Human–dog (Canis familiaris) alignment was 64% identical (Table). Horse exons 2 and 3 were substantially shorter than human, respectively, by 36 and 39 nucleotides. Exon 5 was 6 nucleotides shorter. Each nucleotide sequence was subjected to translation with the ExPASy Translate tool. The 5′3′ frame 3 horse translation lacked internal stops. It also displayed two matches from human lacritin protein versus horse BLASTX screens, and a single mass spec hit (brown underline; Fig. 1B) from horse tears. Overall identity with human lacritin was 45%. Comparative protein identities with cat, dog, and chimp lacritin were, respectively, 38%, 35%, and 99% (Table).
Figure 1. .
Comparative alignment of human lacritin with its horse ortholog. (A) Nucleotide sequence alignment. Horse exons were extracted from Ensembl genomic alignment of human LACRT gene with high-quality draft horse genome (EquCab2.0). AceView directed exclusion of expected untranslated sequence guided the extraction. Horse exons were assembled into a single coding sequence for ClustralW comparison to human. Asterisks indicate identity (75%). Exons are color coded. (B) Protein sequence alignment. Joined horse exons were translated using the ExPASy Translate tool. Only the 5′3′ frame 3 translation lacked internal stops; it also displayed two matches from human lacritin protein versus horse BLASTX screens and the single mass spec hit (brown underline) from horse tears. Boxed is the signal peptide, as predicted by SignalP (4.0). The respective antigens for generation of human anti-N- and anti-C-terminal lacritin antibodies are indicated respectively by (i) and (ii).
Table. .
Identity versus Human Lacritin (417 Nucleotides/138 Amino Acids)
|
Species |
Nucleotide # |
Amino Acid # |
Nucleotide Identity (%) |
Amino Acid Identity (%) |
| Chimp | 414 | 137 | 99 | 99 |
| Horse | 336 | 111 | 75 | 45 |
| Dog | 368 | 118 | 64 | 35 |
| Cat | 356 | 119 | 72 | 38 |
Clustral W2 analysis.
We analyzed horse lacritin in detail for predicted modifications and structure that, when compared to human lacritin, might shed light on function. Like human lacritin, it is predicted to be a secreted protein with a signal peptide that is cleaved between amino acids 19 and 20 (SignalP, Fig. 1B). Human lacritin's C-terminal shared mitogenic and SDC1 binding domain resides within amino acids 100 to 109,2 coded by exon 5. The homologous sequence is GGTELLRKLR from GGTELLRKLRDKFIPIKP (Fig. 1B) that by PSIPRED (version 3.0) is largely alpha helical (underlined; Fig. 2A single asterisk), and by PEPWHEEL predicted to arrange in an amphipathic alpha helix (Fig. 2C). GGTELLRKLRDKFIPIKP also contains putative hydrophobic face leucines (L) and phenylalanine (F; bold) and two to three available lysines (K; italic) in the putative hydrophillic face. In bold and italics are respective hydrophobic face L108, L109, and F112 amino acids involved in ligating SDC1. Cationic face Glu103, Lys107, and Lys111 are thought also to bind SDC1 (Zhang Y, et al., manuscript in preparation, 2012). GTELLRKLRDKFIPIKP also contains hydrophobic face leucines and phenylalanine (bold) and two or three available lysines (italic) in the cationic face. As with human lacritin, other C-terminal α-helices are also predicted, as well as O-glycosylation within the N-terminal half (Fig. 2A, NetOGlyc 3.1). PONDR analysis of order and disorder suggests a less ordered C-terminus than in human lacritin (Fig. 2B), despite PSIPRED-predicted α-helices. These data point to a horse tear ortholog of human lacritin15 with protein identity exceeding that of cat and dog, and with conserved structural features.
Horse Tears
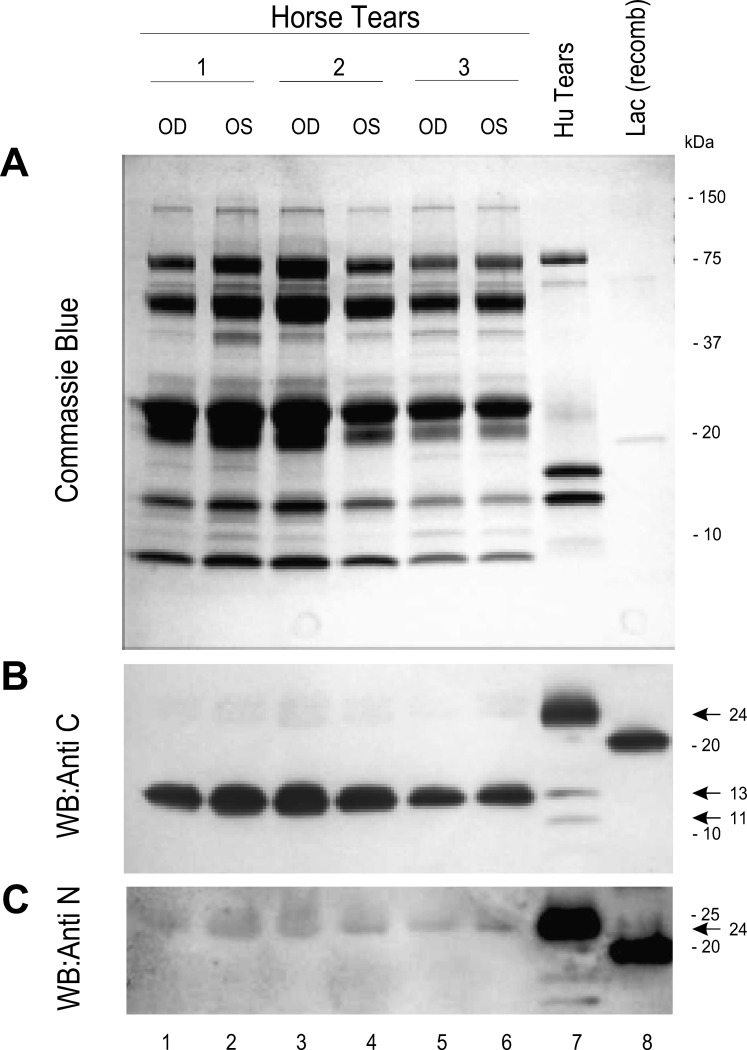
Little is known about the biochemistry and physiology of horse tears, although constituents include connective tissue growth factor, matrix metalloproteinases, and Igs.16,17 We stained SDS-PAGE–separated human and horse tear proteins with Coomassie blue (Fig. 3A, Supplementary Fig. S1, http://www.iovs.org/content/53/10/6130/suppl/DC1). At the loading level utilized, human tears were predominated by bands of ∼85, 16, and 13 kDa, with minor bands of ∼70, 24, and 8.5 kDa. Horse tears displayed a larger variety of proteins (Fig. 3A, lanes 1–6), perhaps related to the method of sponge collection that involved some contact with the ocular surface epithelium and lid. Major bands of ∼85, 45, 26, 22, 13, and 8 kDa were apparent, as well as minor bands of ∼130, 70, 60, 47, 40, 33, 18, 11, and 10 kDa. No differences were apparent between left (OS) and right (OD) eyes, nor between horses.
Figure 3. .
Immunodetection of lacritin in horse tears. (A) Right (OD) and left (OS) eye tear samples from three horses, a human tear sample (Hu Tears), and recombinant human lacritin [Lac(recomb)] were separated by SDS-PAGE and then Coomassie blue stained. Many proteins are apparent in horse tears, some in molecular weight regions not commonly seen in human tears. (B) Horse and human tear samples and recombinant lacritin were separated by SDS-PAGE and blotted. Blots were incubated with anti-C-terminal lacritin antibodies using peroxidase-conjugated secondary antibody. Bound antibodies were detected by chemiluminescence. Lacritin detected in horse tears is smaller than that in human tears and may be a C-terminal fragment. (C) Blotted horse and human tear samples and recombinant lacritin were incubated with anti-N-terminal lacritin antibodies using peroxidase-conjugated secondary antibody and chemiluminescent detection. A band corresponding in size to human tear lacritin was very weakly detected.
Lacritin C- versus N-Terminal Analysis
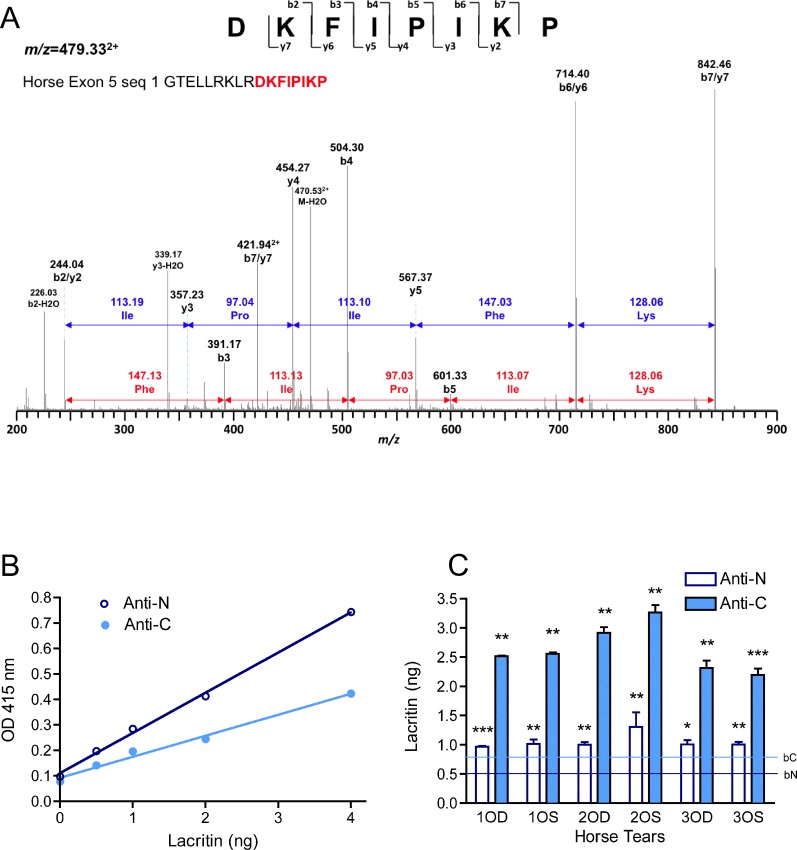
Human lacritin is most strongly expressed by the lacrimal gland for release into tears.1,18 Other lacritin contributors include the meibomian gland19 and corneal and conjunctival epithelia.9 We examined formalin-fixed and paraffin-embedded horse lacrimal gland sections that had been incubated with anti-lacritin antibodies. Slight ductal staining seemed to be present (not shown)—the location of lacritin in human salivary gland.1 Further investigation will require use of frozen sections. To determine if lacritin is detectable in horse tears, SDS-PAGE–separated tear proteins were separately exposed to antibodies directed against the C (anti-C; Fig. 3B)- or N (anti-N; Fig. 3C)-termini of human lacritin. Anti-C was generated against lacritin deletion fragment N-65, a region (Figs. 1, 2) of lacritin derived in part from exon 5. N-Term Lac Pep (Figs. 1, 2), the anti-N antigen, corresponds to sequence largely derived from exon 2, although flanking amino acids Glu(20) and Ser(38) derive respectively from exons 1 and 3. Twelve amino acids of the 19 amino acid N-Term Lac Pep sequence are lacking from horse lacritin (Fig. 1B). Anti-C-term strongly detected native 24 kDa lacritin in human tears. Lacritin C-terminal proteolytic fragments of ∼13 and 11 kDa were also apparent as minor bands. In contrast, the only detected band in horse tears was 13 kDa. Recombinant lacritin generated in E. coli, included as a positive control, was 21 kDa (Fig. 3B). When the 13 kDa horse band was excised and subjected to mass spectrometric sequencing, we obtained DKFIPIKP (Fig. 4A) from GTELLRKLRDKFIPIKP (Fig. 1B) as the sole hit. TBLASTN against the translated horse genome (TaxId: 9796) using default settings (expect threshold 1; word size 3; BLOSUM62 matrix; low complexity regions filter on) revealed no significant similarity; however, DKFIPIKP is shorter than the optimal ≥15 amino acid probe sequence. Using altered settings suggested by National Center for Biotechnology Information for 5 to 15 amino acid probes (expect threshold, 20,000; word size 2; the more stringent PAM30 matrix; low complexity regions filter off; http://www.ncbi.nlm.nih.gov/blast/Why.shtml, in the public domain), no exact hits were obtained over DKFIPIKP's eight amino acids, although some were partially homologous, as would be expected. A bacterial sequence might be the source; however, another search against the entire nr database also yielded no full-length hits. Anti-N-term strongly detected 24 kDa lacritin in human tears. The same-sized protein was the only band apparent in horse tears with anti-N-term (Fig. 3C), although the level of detection was at or barely above background. We turned to ELISA (Figs. 4B, 4C). Standard curve analysis suggested that anti-N had somewhat higher titer for recombinant lacritin (Fig. 4B). Nonetheless, in keeping with Western blotting, anti-C detected lacritin immunoreactivity in horse tears much more effectively (Fig. 4C). All were significantly above background. Average lacritin levels detected per well were, respectively, 2.6 ± 0.4 and 1.1 ± 0.1 ng for anti-C and anti-N minus respective backgrounds of 0.76 ng and 0.53 ng, yielding final values of 1.84 and 0.57 ng per well. These values represent 0.184% and 0.57% of total tear proteins, assuming high coating efficiency, in keeping with coating levels far below saturation.20 Taken together, it is apparent that (1) lacritin is a constituent of horse tears and (2) the majority of horse tear lacritin may exist as a large C-terminal fragment; additionally, (3) analysis suggests that the fragment may be in part structured as an amphipathic α-helix as with the C-terminus of human lacritin. If so, it may share the same SDC1 and mitogenic activities as human lacritin.
Figure 4. .
Mass spectrometric analysis and ELISA quantitation of horse lacritin protein. (A) Mass spectrometric analysis was performed on the 13 kDa band from horse tears. The peak at m/z = 479.332+ was sequenced by MSMS as DKFIPIKP (from horse lacritin GTELLRKLRDKFIPIKP). (B) Plastic wells of 96-well plates were incubated with increasing amounts of recombinant human lacritin at sufficiently low levels for complete adsorption.12 Detection was with anti-C- and -N-terminal lacritin antibodies using peroxidase-conjugated secondary antibody and OPD detection. Standard curves of anti-C- and -N-terminal absorbance (R2 = 0.99275, 0.98739) were developed. (C) Plastic wells of 96-well plates were incubated with sufficiently low levels of horse tears for almost complete expected adsorption. Coated wells were treated with anti-C- and -N-terminal lacritin antibodies with secondary antibody and OPD detection. Absorbance levels were adjusted with standard curves to approximate nanogram levels of lacritin protein. Significance compares amounts to background. *P < 0.01–0.05; **P < 0.01–0.001; ***P < 0.001.
Discussion
We provide evidence for lacritin in horse tears. As rationale, we noted that human,2,8 monkey (Fujii A, et al., IOVS 2011;52:ARVO E-Abstract 3714), rabbit,3 rat,1 and mouse (Walton SC, Laurie GW, unpublished data, 2004) lacrimal or ocular cells are responsive to human recombinant lacritin1–3,8 or lacritin purified from monkey tears (Fujii A, et al., IOVS 2011;52:ARVO E-Abstract 3714), suggesting conservation of lacritin's cell targeting machinery.9 Also Ensembl genomic alignments have uncovered orthologs in cat, rodents (common and northern tree shrew), and others,5 suggesting that lacritin may be common to other mammals. Horse lacritin's size of 13 kDa and predicted structure are similar to those of a C-terminal lacritin fragment detectable in human tears that bears both mitogenic1,2,8 and bactericidal (McKown RL, et al. IOVS 2010;51:ARVO E-Abstract 4181) activities. The implication is of potential benefit in corneal wound repair and dry eye—both significant problems in horses and human.
Apparent tear protein concentration in horses was approximately 10-fold greater than in human tears. This is in keeping with findings by Davidson et al.21 (13.7 mg/mL), who compared β-mercaptoethanol–reduced protein profiles in horse, cow, dog, and rabbit tears collected with microcapillary tubes. Horse, cow, and dog tears all shared bands of 12.9 and 23.5 kDa, putatively lacritin C-terminal fragment and intact lacritin, respectively; but rabbits differed, with comparable bands of 11.6 and 19.7 kDa.21 Other proteins were largely similar in size to ours. Nakajima et al.9 suggested that lacritin was absent from Coomassie blue–stained rabbit, dog, and rat tears; however, assuming partial proteolysis, a similar conclusion might have been reached in the absence of anti-C-terminal anti-lacritin antibodies, particularly with the aberrant mobility of lacritin in SDS-PAGE. The predicted molecular weights of secreted human lacritin and C-terminal fragment N-65 are respectively 12.3 and 5.81 kDa vs. 24 and 13 kDa in SDS-PAGE. Horse lacritin has a predicted molecular weight of 9.9 kDa. A C-terminal fragment equivalent to human N-65 is 5.99 kDa, vs. 13 kDa in SDS-PAGE (assuming conservation of the cleavage site).
Aberrant mobility can be generated by amphipathic α-helices. Apparent conservation of this feature in horse raises the possibility of syndecan-1 cell surface targeting. GTELLRKLRDKFIPIKP's paired leucines and proximal phenylalanine all appear to be essential elements for the ligation of syndecan-1. Syndecan-1 is widely distributed in the animal kingdom, from the fruit fly to humans, with 34 orthologs now known. Heparanase cleavage of syndecan-1′s heparan sulfate is essential for lacritin ligation8 and is also well conserved (fish through to human; 44 orthologs). The third component, the putative signaling receptor(s), is largely unknown. By appropriating novel combinations of preexisting but conserved receptor/coreceptor mechanisms or enzymes, human lacritin is able to ligate nonhuman targets at pharmacological or near-physiological doses. Human or horse lacritin might be appropriate for the treatment of corneal ulcers, a common ophthalmologic problem in horses,22,23 and also for dry eye.
Conclusions
Lacritin is a constituent of horse tears, largely as an ortholog of the C-terminal fragment of human lacritin. In humans, this region is both mitogenic and bactericidal. If similarly active in horses, as predicted from structural studies, the fragment may aid corneal wound repair and dry eye.
Supplementary Material
Acknowledgments
The authors acknowledge the generous supply of human tears from Denise Ryan, Kay Sia, and Scot Bower (Walter Reed Army Medical Center). The authors thank Katherine Spenler and Ryan Brooks (Virginia Tech MARE Center) for their assistance with the collection of equine tears.
Footnotes
Supported by NIH Grants RO1EY013143 and RO1EY018222 (GWL) and the Virginia Commonwealth Health Research Board (RLM).
Disclosure: D.E. Laurie, None; R.K. Splan, None; K. Green, None; K.M. Still, None; R.L. McKown, EyeRx (E, S), P; G.W. Laurie, EyeRx (C), P
References
- 1.Sanghi S, Kumar R, Lumsden A, et al. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol. 2001;310:127–139 [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Wang N, Xie J, et al. Restricted epithelial proliferation by lacritin via PKCα-dependent NFAT and mTOR pathways. J Cell Biol. 2006;174:689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samudre SS, Lattanzio FA, Lossen V, et al. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthalmol Vis Sci. 2011;52:6265–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009;28:1109–1117 [DOI] [PubMed] [Google Scholar]
- 5.McKown RL, Wang N, Raab RW, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88:848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo BS, Lee DY, Ha HS, Kim JC, Kim CW. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Proteome Res. 2005;4:719–724 [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan S, Thangavelu M, Zhang L, Green KB, Nichols KK. iTRAQ quantitative proteomics in the analysis of tears in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5052–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma P, Beck SL, Raab RW, et al. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J Cell Biol. 2006;174:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima T, Walkup RD, Tochigi A, Shearer TR, Azuma M. Establishment of an appropriate animal model for lacritin studies: cloning and characterization of lacritin in monkey eyes. Exp Eye Res. 2007;85:651–658 [DOI] [PubMed] [Google Scholar]
- 10.Utter ME, Davidson EJ, Wotman KL. Clinical features and outcomes of severe ulcerative keratitis with medical and surgical management in 41 horses (2000–2006). Equine Vet Educ. 2009;21:321–327 [Google Scholar]
- 11.Wade CM, Giulotto E, Sigurdsson S, et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science. 2009;326:865–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert K, Gandia NC, Wilburn JK, et al. Tear lacritin levels by age, gender, and time of day in healthy adults. Invest Ophthalmol Vis Sci. 2012. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Freitas MA. A mass accuracy sensitive probability based scoring algorithm for database searching of tandem mass spectrometry data. BMC Bioinformatics. 2007;8:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanghi S, Kumar R, Walton S, Laurie GW. Quantitation of rat lacrimal secretion: a novel sandwich ELISA with high sensitivity. Exp Eye Res. 2000;70:651–658 [DOI] [PubMed] [Google Scholar]
- 15.Ozyildirim AM, Wistow GJ, Gao J, et al. The lacrimal gland transcriptome is an unusually rich source of rare and poorly characterized gene transcripts. Invest Ophthalmol Vis Sci. 2005;46:1572–1580 [DOI] [PubMed] [Google Scholar]
- 16.Martin E, Molleda JM, Ginel PJ, Novales M, Lucena R, Lopez R. Total protein and immunoglobulin concentrations in equine tears. J Vet Med. 1997;44:461–465 [DOI] [PubMed] [Google Scholar]
- 17.Strubbe TD, Brooks DE, Schultz GS, et al. Evaluation of tear film proteinases in horses with ulcerative keratitis. Vet Opthalmol. 2000;3:111–119 [DOI] [PubMed] [Google Scholar]
- 18.Morimoto-Tochigi A, Walkup RD, Nakajima E, Shearer TR, Azuma M. Mechanism for carbachol-induced secretion of lacritin in cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2010;51:4395–4406 [DOI] [PubMed] [Google Scholar]
- 19.Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen K, Brobeck U. Assessment of coating-efficiency in ELISA plates by direct protein determination. J Immunol Methods. 1986;95:291–293 [DOI] [PubMed] [Google Scholar]
- 21.Davidson HJ, Blanchard GL, Montgomery PC. Comparisons of tear proteins in the cow, horse, dog and rabbit. Adv Exp Med Biol. 1994;350:331–334 [DOI] [PubMed] [Google Scholar]
- 22.Brünott A, Boevé MH, Velden MA. Grid keratotomy as a treatment for superficial nonhealing corneal ulcers in 10 horses. Vet Ophthalmol. 2007;10:162–167 [DOI] [PubMed] [Google Scholar]
- 23.Moore PA. Diagnosis and management of chronic corneal epithelial defects (indolent corneal ulcerations). Clin Tech Small Anim Pract. 2003;18:168–177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.