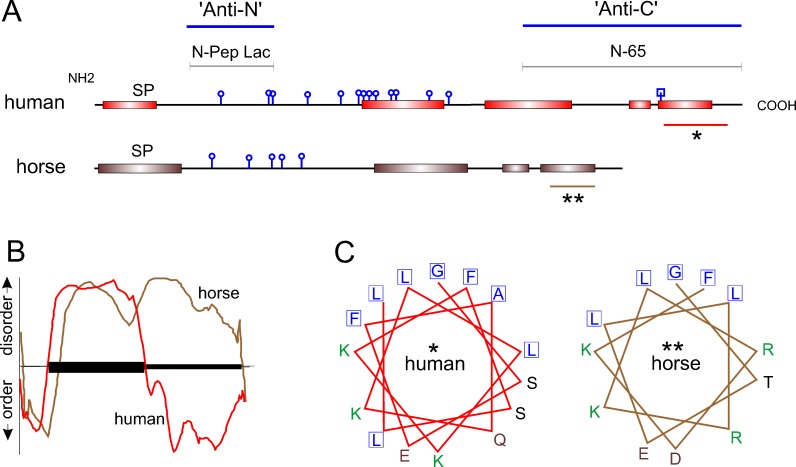
Figure 2. .
Comparative analysis of predicted secondary structure. (A) NetOGlyc 3.1 predicts at least five O-linked glycosylation sites (round knobs) in horse versus 13 in humans. N-linked glycosylation centered on asparagine 119 in human lacritin is predicted by NetNGlyc 1.0 (square knob). PSIPRED (version 3.0) predicts several C-terminal α-helices in horse lacritin, much like human. The two large C-terminal helices in human lacritin have been validated by circular dichroism2 (Beck SL, Laurie GW, unpublished data, 2005). The human N-Pep Lac peptide and recombinant truncation mutant N-65 are antigens for anti-N- and anti-C-terminal lacritin antibodies noted in Figure 1. SP, signal peptide. Asterisks indicate regions further analyzed. In human lacritin, the C-terminal α-helix (*) forms the syndecan-1 binding domain for cell targeting.8 (B) PONDR analysis of human and horse lacritin sequences. The y-axis indicates predicted disorder and order, respectively, above and below the x-axis. Protein length is represented along the x-axis with the amino terminal signal peptide indicated by the ordered domain at left. The slightly longer human lacritin tracing has been compressed to fit on the same x-axis scale with the horse. PONDR predicts that human lacritin may contain more C-terminal order than horse lacritin. (C) PEPWHEEL analysis of α-helical regions indicated by asterisks in (A). The distinctive hydrophobic face (adjacent boxed residues) of this region in human lacritin2 is partially conserved in horse lacritin. The human hydrophobic face is required for syndecan-1 binding, a function predicted to be shared by horse lacritin. Amino acids with nonpolar, basic, acidic, and uncharged side chains are respectively blue, green, brown, and black.