Abstract
Australasian gannets (Morus serrator), like many other seabird species, locate pelagic prey from the air and perform rapid plunge dives for their capture. Prey are captured underwater either in the momentum (M) phase of the dive while descending through the water column, or the wing flapping (WF) phase while moving, using the wings for propulsion. Detection of prey from the air is clearly visually guided, but it remains unknown whether plunge diving birds also use vision in the underwater phase of the dive. Here we address the question of whether gannets are capable of visually accommodating in the transition from aerial to aquatic vision, and analyse underwater video footage for evidence that gannets use vision in the aquatic phases of hunting. Photokeratometry and infrared video photorefraction revealed that, immediately upon submergence of the head, gannet eyes accommodate and overcome the loss of greater than 45 D (dioptres) of corneal refractive power which occurs in the transition between air and water. Analyses of underwater video showed the highest prey capture rates during WF phase when gannets actively pursue individual fish, a behaviour that very likely involves visual guidance, following the transition after the plunge dive's M phase. This is to our knowledge the first demonstration of the capacity for visual accommodation underwater in a plunge diving bird while capturing submerged prey detected from the air.
Keywords: amphibious vision, underwater accommodation, corneal power, foraging tactics, gannets, visual prey detection
1. Introduction
Many vertebrates regularly alternate their activities between air and water [1]. The need to function in both media, at the sensory and motor levels, imposes major constraints, evolutionary pressures and physiological trade-offs on the individual's morphology, physiology and sensory systems [2]. In the face of these opportunities and constraints, many species, among them piscivorous birds, successfully perform fine-tuned sensory and motor tasks in both media.
Piscivorous birds may be grouped into two categories based on their foraging patterns. One group comprises birds that search for aquatic prey from the air, and capture it using rapid motions such as bill-strikes (e.g. herons (Ardeidae)), or plunge-dives (e.g. kingfishers (Cerylidae), terns (Sternidae), fish eagles (Accipitridae) and osprey (Pandionidae)). The second group both detects and captures fish underwater, after submergence of their eyes (e.g. penguins (Spheniscidae), auks and guillemots (Alcidae) and cormorants and darters (Phlacrocoridae and Anhingidae, respectively)) [3]. Common to both groups, however, are certain aspects of their visual ecology, including prolonged exposure to reflected sunlight and skylight rich in short wavelengths and continuous changes of intensity (glitter/shimmer) due to water surface motion [4,5]. The eyes of birds in both groups must therefore be shaped by similar and different environmental pressures.
Birds that plunge-dive or strike at fish perform visual detection and location of submerged prey from the air under complex optical conditions, including variation in the reflection and refraction of light [6–9]. Visual constraints in birds that pursue their prey underwater extend to the dioptrics of the eye as well as to differences in photic environments. The avian eye has primarily evolved to perform in air, and the quality of the image formed on the retina is determined predominately by the cornea and to a lesser extent by the lens [10]. The cornea in air is bordered on its inner surface by the aqueous humour, with a refractive index of 1.33, and on its outer surface by air, with a refractive index of 1.0 [11]. Under these conditions, the cornea is the principal refracting agent of light rays and is responsible for approximately two-thirds of the refractive power of the eye. Underwater, the media bathing the inner and outer surfaces of the cornea (the aqueous humour and water, respectively), are of similar refractive indices, and the refractive power of the cornea is thus lost, leaving the lens as the sole agent for visual accommodative adjustments [12].
For the image to remain sharp on the bird's retina upon submergence, the lens must be capable of providing the refractive power lost by the cornea [13–18]. Because the refractive power of the cornea and the lens are a function of the curvature of its surfaces, lenses of fish [18], amphibians [19], penguins [20], cetaceans [21] and seals [22] have evolved to be spherical and, thus, provide maximal refractive power.
It has been found that mergansers (Anatidae), cormorants (Phalacrocoracidae) and other underwater pursuit-diver birds also have strongly curved corneas, and experiments indicate a pronounced capacity for lenticular accommodation [17,23,24], although there is still no agreement on the muscular mechanisms involved. The refractive power of the cornea of great cormorants, Phalacrocorax carbo sinensis, in air is ca 50 D and when they voluntarily submerge their eyes they maintain emmetropia (in less than 20–40 ms), i.e. they fully compensate for the loss of this amount of corneal refractive power [11].
Gannets (Morus spp., Sulidae) capture pelagic prey (fish and squid) by plunge diving into the sea from heights often exceeding 5 m [25–27]. Once submerged, they either adopt a V-shaped dive profile, in which the bird surfaces immediately after a downward M phase of the plunge that occasionally includes a short phase of wing flapping (WF), or a U-shaped profile in which the M phase is followed by a longer phase of active propulsion using wing beats [28,29]. This provides for flexible underwater hunting tactics [28], where gannets are able to use the speed of M or switch their feeding style to active pursuit using WF [27,30–32].
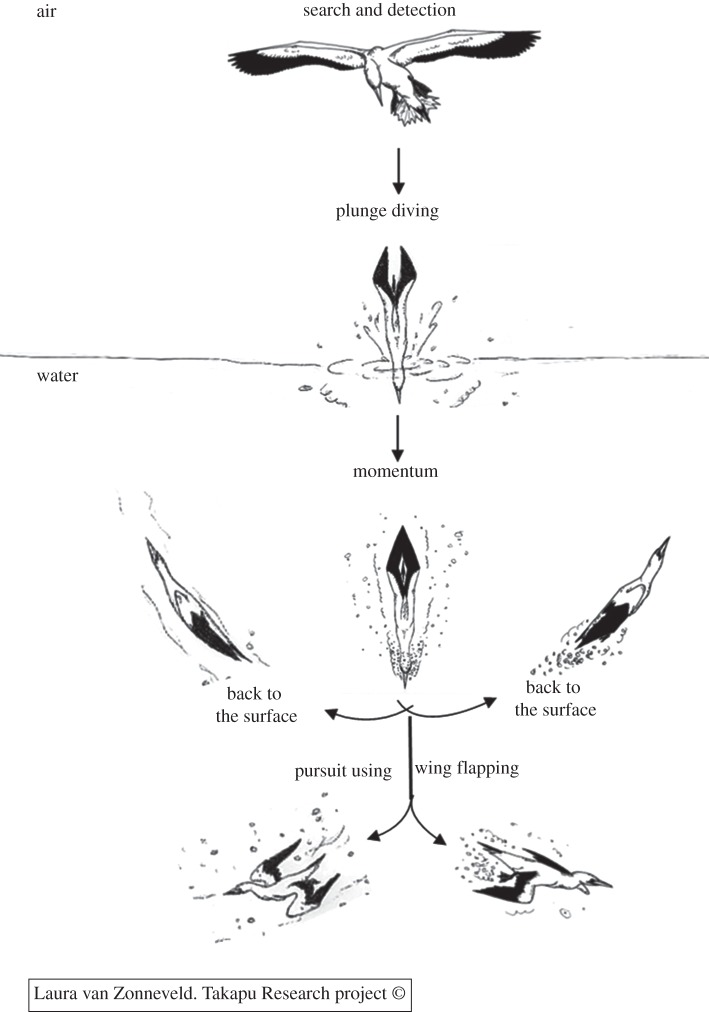
The detection of prey from the air [33–36] is regarded as visually guided. However, evidence that vision is the sensory modality used during active pursuit of prey underwater has been provided only for the detection and pursuit of prey in great cormorants, a species that does not plunge dive [37–40]. At the optical, visual and photic levels the search and detection, plummeting and underwater M phases of the dives of gannets are similar to patterns observed in other plunge divers or strikers, such as kingfishers (Alcedinidae) or herons, whereas WF is similar to that observed in pursuit divers, such as cormorants (figure 1). Gannets thus face two major visual obstacles, related to the air–water interface and to amphibious accommodation, distinguishing gannets' foraging tactics from other seabirds.
Figure 1.
Dive patterns of Australasian gannets: Prey capture in the momentum (M) and in the wing flapping (WF) phases.
Here we examine the potential role of underwater vision in Australasian gannets while plunge diving, using infrared (IR) photorefraction, photokeratometry and underwater videography. Our aims were to: (i) establish if Australasian gannets are capable of visual accommodation underwater, and if so, measure the amount of corneal refractive power that is overcome and (ii) search for behavioural evidence that gannets use aquatic vision in hunting by analysing underwater video footage of foraging gannets.
2. Material and methods
(a). Analysis of underwater video footage
A total of 55 min of underwater video footage (at 30 frames per second) of Australasian gannet foraging associated with stationary prey balls that were formed by the presence of dusky dolphins (Lagenorhynchus obscurus) was analysed frame by frame using Adobe Premiere Pro CS4. The footage was collected between 24 August and 31 October 2005, 8–12 August 2006 and 17 September 2009 in Admiralty Bay and Current Basin in the Marlborough Sounds, New Zealand. For the analysis, a dive was considered to be the period from the time that the gannet penetrated the water to its return to the surface. In the 95 dives analysed, prey capture was observed both in the underwater M phase of the dive, in which the gannets descend through the water column without wing propulsion, and in the WF phase, in which gannets are propelled through the water by active wings movement (figure 1). To evaluate the role of each phase in hunting, we quantified the number of successful prey captures and the rate of prey capture (prey captured per time in the dive) during the M and WF phases. Data were statistically tested using χ2 and t-test (PASW Statistics v. 18).
(b). Visual accommodation in air and underwater
The states of underwater accommodation were determined based on photokeratometry and on IR photorefraction. Photokeratometry is a photographic method of determining the curvature of the cornea and hence its refractive power. The photokeratometer used was essentially that described previously [15,41]. It consisted of a Canon EOS-10D SLR camera with a Canon EF 35 mm 1 : 2 lens operated at full aperture for minimum depth of field. A light ring (Zeiss) was mounted on the camera's objective lens with ca 20 apertures, each less than 0.5 mm in diameter, forming a circle of a radius of 35 mm around the lens optic axis. The camera flash (Woctron-250PC-Auto) was projected via an optic fibre to the light ring. For calibration, we used a set of five steel ball bearings, 8, 10, 15, 20 and 25 mm in diameter. Each ball was measured to the nearest 0.1 mm using Vernier calipers and was photographed with the photokeratometer mounted on a tripod. The focus of the camera lens was set at infinity and in taking the photographs, the camera-to-ball distance was adjusted for the sharpest image. For each ball, the distances between opposite reflections of the keratometric reflection circle were determined from three readings, approximately along the 0°, 45°, 90° and 135° meridians. The mean of the measurements was calculated, and we regressed the ball bearing diameters against the mean reflection distances measured on the film plane. The resultant regression equation was used to estimate the corneal radii (i.e. half of the diameters of the calibration ball bearings) of gannets' eyes in the field.
The dioptric power of a cornea (F, in dioptres, D) was determined by the equation: F = 337.5/R, where R is the corneal radius (measured in millimetres). This equation expresses the power of the human cornea as a function of the radius of its first surface [42]. The diameters of the light rings reflected off the examined corneas were measured from photographs (un-edited) of eyes taken in the field. All photographs with a sharp image of the reflected ring of light were used in the analyses. The diameter of each reflected ring was measured and the respective corneal diameter calculated from the regression equation mentioned earlier.
Eye photography was performed at the Cape Kidnappers colony (39°38′ S, 177°05′ E) during 2011, under permission of the New Zealand Department of Conservation (ECHB-23237-RES) and of the Massey University Ethics committee (09/76). A bird to be measured was captured by an experimenter at the periphery of the colony, using a shepherd's hook, and then restrained by hand. Using the photokeratometer, the second experimenter took ca five photographs of one eye, followed by ca five photographs of the contra-lateral eye (figure 2). In taking the photographs, the camera-to-bird distance was adjusted for the sharpest image. Pronounced eye movements and rapid flicking of the nictitating membrane resulted in a proportion of the digital images being unsuitable for analysis. For each eye of each bird, the two photographs that provided the sharpest and best-centred images of the photokeratometric light reflections were used for extracting the values of the distances between opposite reflections along the four meridians.
Figure 2.
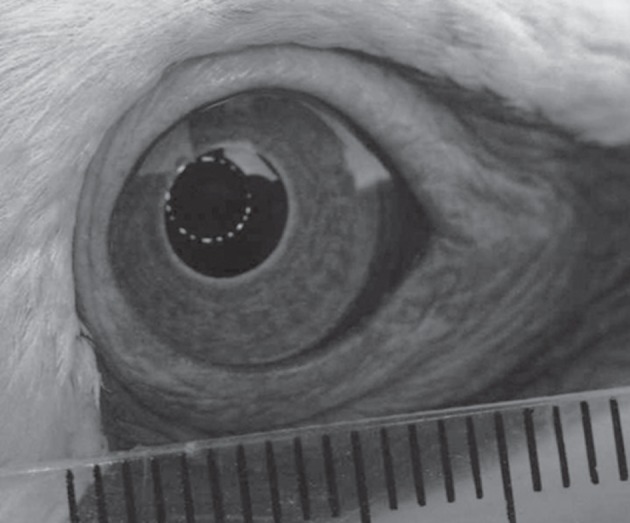
The eyes of a gannet, photographed with a photokeratoscope and showing the light ring reflected off the cornea. Scale bar, 1 mm.
Photorefraction was performed using an IR video retinoscope to measure their natural accommodation. The underlying principles of the system are provided in detail in Schaeffel et al. [43]. In brief, the IR retinoscope is based on a light source adjacent, and eccentric, to a video-camera lens’ axis that projects light rays parallel to the camera's axis and records the reflection from the fundus. The use of IR minimizes disturbance to the animals. The light reflected off the fundus appears as a crescent in the pupil, and the position of the reflex indicates the sign of the defocus relative to the camera. In hyperopia, the reflex appears at the top of the pupil, while in myopia the reflex appears at the bottom of the pupil. The amount of defocus (D) is obtained from the size of the reflex: D = E/(2ADFR), where E is the eccentricity of the light source, A is the distance of the camera to the eye, DF is the dark fraction in the pupil and R is the pupil radius (all units in metres or dioptres, i.e. reciprocal metres). To improve the precision of the measurements, the light sources (LEDs) are set in five rows, providing five different eccentricities (2, 6, 10, 14 and 18 mm). LED illumination was either in a temporal sequence, providing consecutively five different crescents, or set to a given eccentricity only. During the field tests we used both methods. Owing to a certain ambiguity of the precise eccentricity of the light at specific instances, the calculations of defocus here were for the eccentricity of 10 mm.
The IR retinoscope comprised a CCD camera (Watec LCL902K; 30 Hz), with a Nikon lens (55 mm/1 : 1.2) fitted with a supplementary lens. The video camera was connected to a Toshiba laptop and the images captured using Movie-Maker. Owing to the mobility of the birds’ head and eyes, no attempt was made to verify the amount of defocus by the use of correction lenses.
Tests were conducted over two consecutive days in March 2011. The IR retinoscope, on a tripod, was positioned ca 1.0–1.2 m from the front wall of the experimental Perspex aquarium, with the camera's optical axis perpendicular to the wall. The aquarium (80 × 40 × 50 cm; length × width × height) was kept three-quarters full of water. The setup was placed ca 20 m from the edge of the gannet colony, and measurements were performed under natural low light levels (ca 0.01 Lux), to minimize stress to the birds and to achieve maximal pupil opening and thus enhance the IR effect.
Test birds were captured at the periphery of the colony using a shepherd's hook and transferred by hand to the setup. The experimenter, holding the gannet, aligned the bird's head so that its bill pointed ca 45° downwards. Then, in a single smooth motion, the experimenter submerged the gannet's head in the water for 2–5 s, to a depth of water ca 10 cm above the eye and ca 5–10 cm from the aquarium's wall. The second investigator, positioned so as to view the aquarium's long axis and level with the water surface, filmed the bird from when it was ca 50 cm above the water level to the end of its submergence. We moved the bird towards the aquarium and submerged its head in the plane parallel to the aquarium wall. For all birds, only the left eye was filmed and filming was conducted when the bird was ≈1.0–1.2 m from the camera lens providing an optical distance, i.e. (distance in air + distance in water/1.33) of ca 1.0 m. Selected video sequences were captured using Adobe Premier v. 6.0 to determine states of accommodation. Once tested, the bird was immediately released at the colony edge nearest its capture site.
(c). Evaluation of the individual video frames in optical analysis
Images of individual frames from the video recordings were transferred to Photoshop for measurement using pixel counting. We calibrated pixel dimensions and pupil size in underwater frames by measuring the width of the base of the bill from the frames taken in air, where a ruler was included in the picture for scale, as well as the corresponding bill width in underwater pictures. We then scaled the underwater pupil sizes accordingly. Measurements were made to the nearest pixel (representing approx. 0.11 mm in air and 0.29 mm in water). The darkened portion of the pupil was also measured, the dark fraction of the pupil was calculated and the data entered into equation already mentioned (F = 337.5/R), using the relevant values of eccentricity. Because, in photorefraction, myopic illuminated crescents appear in the pupil on the same side as the light source, while hyperopic crescents appear on the opposite side, it was easy to distinguish hyperopic and myopic reflexes.
3. Results
(a). Diving behaviour
Ninety five dives were analysed from the behavioural video footage. Results showed that the duration of the M phase (n = 95, 0.85 ± 0.035 s) was significantly shorter than the WF phase (n = 81, 5.94 ± 0.44 s) (t = 11.398; d.f. = 80; p < 0.0001; two-tailed paired t-test). Additionally, significantly more successful prey captures were observed in the WF (n = 47) than the M phase (n = 25; χ2 = 24.785; d.f. = 1; p < 0.001; two-tailed test), and the proportion of successful dives was significantly higher in the WF (91.1%) than the M phase (45.5%) (χ2 = −6.936; d.f. = 1; p < 0.001; two-tailed test). Further, more capture attempts were observed in WF (n = 67) than the M phase (n = 58; χ2 = 8.24; d.f. = 1; p < 0.01; two-tailed test). Of the successful dives, in 5 per cent of the events a gannet captured fish during the M phase, and immediately thereafter switched to WF pursuit and captured another fish.
(b). Corneal power
We photographed the eyes of 14 gannets (10 adults of unknown sex and four juveniles) using a photokeratometer. For all birds, at least one sharp image was obtained for each eye. The diameter of the light circle, reflected off the cornea, was 3.34 ± 0.17 mm (mean ± s.e.) in adult gannets and 2.99 ± 0.26 mm in the juveniles. These yielded calculated mean globe diameters of 15.39 mm in the adults and 13.89 mm in the juveniles and a calculated mean corneal refractive power 43.93 ± 2.15 D (dioptres) for adults and 48.93 ± 4.21 D for juveniles.
(c). Pupil size
We measured the size of the gannets' pupils in air, immediately prior to submergence and underwater. Under the low light levels (starlight), the pupils were wide open (figures 3 and 4). Overall, pupil diameter underwater (vertical 8.98 ± 0.81 mm, horizontal 6.51 ± 0.72 mm; mean ± s.e., n = 5) was similar to that in air (vertical: 9.48 ± 0.69; horizontal: 6.75 ± 0.72; n = 5). Furthermore, no apparent differences were observed in pupil diameter between states of hyperopia, emmetropia and myopia (figure 4). In comparison, in air, under direct sunlight, pupil diameter was 4.44 ± 0.38 mm (n = 5).
Figure 3.
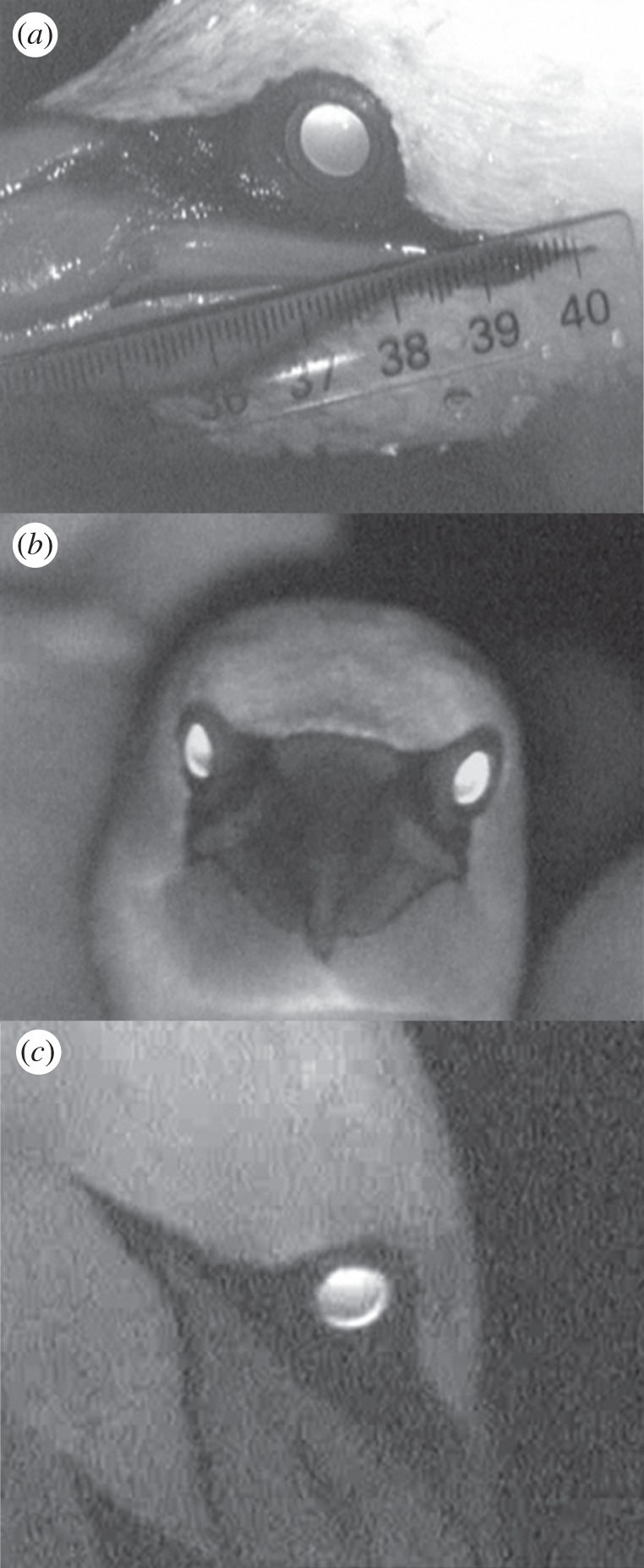
Infrared (IR) light reflected from the eyes' fundus showing (a) a fully open pupil eye in air, in darkness, of a hand restrained gannet, with the higher intensity crescent at the dorsal part of the pupil, indicating a refractive state of hyperopia, (b) an unrestricted gannet in the colony, in air, showing a binocular viewing of the camera, (c) a concentric ring at the periphery of the pupil, that may be indicative of lens multi-focality.
Figure 4.
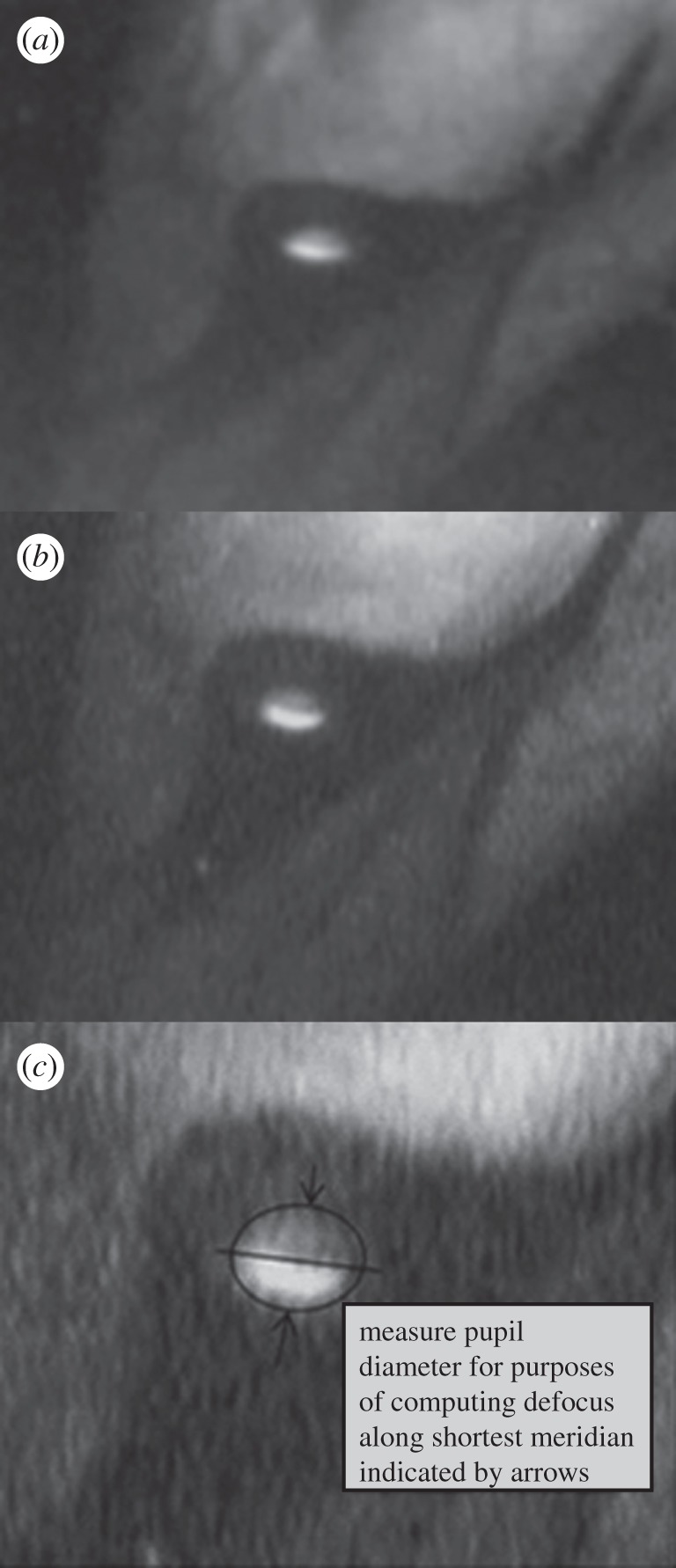
(a,b) The light pattern reflected off the fundus, through the pupil of a gannet's eye underwater. The light crescent of higher intensity at the lower part of the pupil indicates a state of myopia. (c) The procedure employed for determining the dark fraction of the pupil.
(d). Underwater accommodation
In most filmed sequences, the eyes in air and underwater were in a refractive state of hyperopia (figure 4) while images of underwater states of myopia were rare. For five gannets, sharp images were obtained for the determination of pupil size, and two birds were analysed for underwater accommodation. Underwater myopic defocus values (mean, dioptres ± s.d.) calculated for an eccentricity of 10 mm, were, respectively, 9.04 ± 3 D for bird 1 and 9.72 ± 3 D for bird 2.
The results clearly show that, underwater, the gannets are capable of reaching a state of myopia. In so doing, they overcome the loss of corneal refractive power and the focusing demand. The transition from aerial to underwater accommodation is rapid: not infrequently the eye was in a state of hyperopia while the bill was touching the water surface and in a state of myopia at the instant of the subsequent clear underwater image (ca two to three frames, 80–120 ms later), with the entrance of the eye into the water blurred by water spray.
4. Discussion
Kröger [44] noted that ‘It has been a particular challenge to natural evolution to find eye designs that are equally useful in both air and water’ (p.115). In plunge divers such as gannets, it is highly likely that plunges are guided using visual detection of prey from the air [33–36]. Our examination of their underwater hunting behaviour suggests that gannets use vision also in the aquatic phase of the hunt, and our optical analysis has demonstrated some of the mechanisms that have evolved to enable these birds to meet the challenge of rapidly transitioning from aerial to aquatic vision.
As has been shown previously [27,29,31,32], the video footage used in our study clearly distinguished an M phase and WF phase in gannet dives. It has been suggested that capturing prey during the M phase provides gannets the benefit of surprise [45]. However, our results showed that Australasian gannets are more successful in prey capture in the active pursuit (WF) phase than in the M phase of the dives.
What might account for the greater capture success in the WF phase, where the advantage of surprise does not apply? Our analysis suggests that aquatic vision plays a role. In the M phase, the acceleration of flow around a gannet's body associated with the high entrance velocity in the plunge results in pressure dropping locally below the vapour pressure, causing bubbles (cavitation; [46]) markedly affect underwater image quality [47]. Also, body manoeuvrability may be constrained by its high entry speed and cavitation [48]. In contrast, the slower movement in the WF phase enables the use of vision unobstructed by cavitation. That gannets in fact do capitalize on the greater opportunity to use vision in the WF phase is suggested by the rapid directional adjustments the birds make to compensate for evasive movements of prey during the pursuit. The large number of fish, and the extensive mixing of water associated with multi-species feeding events, makes it highly unlikely that olfaction could provide a sufficiently directional cue for such pursuits. The high turbulence would, likewise, greatly limit the use of mechanosensory cues in the pursuit of individual fish. Further, mechanosensory cues could not have been used in the case of underwater kleptoparasitism where gannets specifically targeted fish that had already been immobilized in the beaks of other conspecifics [49]. Overall, this analysis suggests that the WF phase is the main stage in the foraging strategy of gannets.
However, if the image is to remain sharp on the retina (i.e. be emmetropic) upon the gannet's submergence, to allow capturing the fish with the bill, the optics of the eyes should undergo pronounced changes so as to accommodate underwater. Cormorants are capable of large magnitude, rapid accommodation upon head submergence, overcoming loss of corneal power greater than 50 D in ca 40 ms [11]. This is achieved, most probably, through a rapid change in the shape of the lens. Underwater visual acuity of great cormorants, determined behaviourally (ca 9 arcmin) is lower than in air (ca 3 arcmin) and yet remains similar to that of their potential prey fish [37–39]. Our results suggest that Australasian gannets are, similarly, capable of compensating for the loss of refraction at the cornea in water by lenticular accommodation. Gannets' eyes are larger than cormorants' providing a lower corneal curvature and hence a lower refractive power (ca 44 D in gannets compared with ca 50–60 D in cormorants). Corneal refractive power of juvenile gannets was higher than that of adults as their eyes have probably not reached their full size and the lower radius of curvature provided for a higher refractive power [11].
Earlier studies have suggested that the capacity for underwater accommodation in pursuit diving birds such as mergansers and cormorants is brought about by the joint performance of the enlarged iris muscles acting on the highly pliable lens (reviewed in [17]). However, observations on the hand-held gannets in the present study, and of voluntary dives of great cormorants [11], do not support a pupil constriction upon submergence and accommodation. In structure and function, the iris acts as two separate muscles—a peripheral one that constricts the lens and a central one that controls the aperture. Pupil size plays an important role in image formation by governing retinal illumination and depth of field [50]. The wide open pupil aperture underwater, in both gannets and great cormorants, must result in a trade-off between lower resolution and higher image illumination, which is especially important in considering the sharp decline in ambient illumination with dive depth. Underwater accommodation in the gannets was attained within two to three frames (80–120 ms) of submergence, also similar to the velocities observed in cormorants. It may thus be concluded that at the instant of entering the water from heights often exceeding 5 m [25,27], the gannets' optics shifts from aerial to aquatic vision, allowing them to better detect their prey.
Our study has provided the first demonstration of the capacity for visual accommodation underwater in a plunge diving bird, suggesting that Australasian gannets are capable of coping with the optical demands of rapidly transitioning from aerial to aquatic vision. More work is needed to determine how gannets meet the visual challenges associated with the aerial phase of the hunt. In particular, how do they detect fish against a background that undergoes sharp spatio-temporal changes in the intensity of reflected light (glare)? And how do they compensate for refraction-induced image displacement, and cope with the apparent motion of the prey induced by refraction on a moving surface? Answers to such questions will further highlight the reasons why the eye has long been upheld as an exemplar in amphibious predators' optimization through evolution [51].
Acknowledgements
We thank Danny Boulton for loaning some underwater video footage to us. The Department of Conservation (DoC), Napier Office, provided accommodation in the field, and Cape Kidnappers landowners and farm managers kindly gave admission to the property. We also thank Idan Shapira, Dianne Brunton and Uri Shanas for making this collaboration possible and Laura van Zonneveld, Eric Libby and Gary Greyling for assistance in the field and helpful discussions in early stages of the manuscript. Aspects of this work were funded by National Geographic Waitt funding, Massey University International Visitors Research Fund (IVRF) and the Israeli Academy of Sciences (to G.K.). G.M.C. is a recipient of the Institute of Natural Sciences at Massey University (INS) doctoral scholarship. D.R. is part-funded by the National Research Centre for Growth and Development, New Zealand.
References
- 1.Thewissen J. G. M., Nummela S. (eds) 2008. Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates. Berkeley, CA: University of California Press [Google Scholar]
- 2.Kröger R. H. H., Katzir G. 2008. Comparative anatomy and physiology of vision in aquatic tetrapods. In Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates (eds Thewissen J. G. M., Nummela S.), pp. 121–147 Berkeley, CA: University of California Press [Google Scholar]
- 3.Cramp S., Simmons K. E. L. 1977. The birds of the Western Palearctic—handbook of the birds of Europe, the Middle East and North Africa, vol. I. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Lythgoe J. 1979. The ecology of vision. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Loew E. R., McFarland W. N. 1990. The underwater visual environment. In The visual system of fish (eds Douglas R. H., Djamgouz M. B. A.), pp. 1–44 London, UK: Chapman and Hall [Google Scholar]
- 6.Labinger Z., Benjamini Y., Katzir G. 1991. Prey size choice in captive pied kingfishers (Ceryle rudis). Anim. Behav. 42, 969–975 10.1016/S0003-3472(05)80149-6 (doi:10.1016/S0003-3472(05)80149-6) [DOI] [Google Scholar]
- 7.Katzir G., Camhi J. M. 1993. Escape response of Black mollies (Poecilia sphenops) to predatory dives of Pied kingfisher (Ceryle rudis). Copeia 2, 549–553 10.2307/1447160 (doi:10.2307/1447160) [DOI] [Google Scholar]
- 8.Katzir G., Martin G. R. 1994. Visual fields in herons (Ardeidae)—Panoramic vision beneath the bill. Naturwissenschaften 81, 182–184 10.1007/BF01134539 (doi:10.1007/BF01134539) [DOI] [Google Scholar]
- 9.Katzir G., Strod T., Schechtman E., Hareli S., Arad Z. 1999. Cattle egrets are less able to cope with light refraction than are other herons. Anim. Behav. 57, 687–694 10.1006/anbe.1998.1002 (doi:10.1006/anbe.1998.1002) [DOI] [PubMed] [Google Scholar]
- 10.Walls C. L. 1967. The vertebrate eye and its adaptive radiation. New York, NY: Hafner [Google Scholar]
- 11.Katzir G., Howland H. C. 2003. Corneal power and underwater accommodation in great cormorants, Phalacrocorax carbo sinensis. J. Exp. Biol. 206, 833–841 10.1242/jeb.00142 (doi:10.1242/jeb.00142) [DOI] [PubMed] [Google Scholar]
- 12.Sivak J. G. 1980. Avian mechanisms for vision in air and water. Trends Neurosci. 12, 314–317 10.1016/0166-2236(80)90113-7 (doi:10.1016/0166-2236(80)90113-7) [DOI] [Google Scholar]
- 13.Martin G. R. 1998. Eye structure and amphibious foraging in albatrosses. Proc. R. Soc. Lond. B 265, 665–671 10.1098/rspb.1998.0345 (doi:10.1098/rspb.1998.0345) [DOI] [Google Scholar]
- 14.Sivak J. G., Millodot M. 1977. Optical performance of the penguin eye in air and in water. J. Comp. Physiol. A 119, 241–247 10.1007/BF00656636 (doi:10.1007/BF00656636) [DOI] [Google Scholar]
- 15.Howland H. C., Howland M., Guinta A., Cronin T. W. 1997. Corneal curvature and refraction of central American frogs. Vis. Res. 25, 73–81 10.1016/S0042-6989(96)00135-6 (doi:10.1016/S0042-6989(96)00135-6) [DOI] [PubMed] [Google Scholar]
- 16.Howland H. C., Sivak J. G. 1984. Penguin vision in air and water. Vis. Res. 24, 1905–1909 10.1016/0042-6989(84)90024-5 (doi:10.1016/0042-6989(84)90024-5) [DOI] [PubMed] [Google Scholar]
- 17.Glasser A., Howland H. C. 1996. A history of studies of visual accommodation in birds. Q. Rev. Biol. 71, 475–509 10.1086/419554 (doi:10.1086/419554) [DOI] [PubMed] [Google Scholar]
- 18.Land M. F. 1990. Optics of the eyes of marine animals. In Light and life in the sea (eds Herring P. J., Campbell A. K., Whitfield M., Maddock L.), pp. 149–166 Cambridge, UK: Cambridge University Press [Google Scholar]
- 19.Mathis U., Schaeffel F., Howland H. C. 1988. Visual optics in toads. J. Comp. Physiol. A 163, 201–213 10.1007/BF00612429 (doi:10.1007/BF00612429) [DOI] [PubMed] [Google Scholar]
- 20.Sivak J. G., Howland H. C., McGill-Harelstad P. 1987. Vision of the Humboldt penguin (Spheniscus humboldti) in air and water. Proc. R. Soc. Lond. B 229, 467–472 10.1098/rspb.1987.0005 (doi:10.1098/rspb.1987.0005) [DOI] [PubMed] [Google Scholar]
- 21.Mass A. M., Supin A. Y. 2009. Vision. In Encyclopedia of marine mammals (eds Perrin W. F., Würsig B., Thewissen J. G. M.), pp. 1200–1211, 2nd edn San Diego, CA: Elsevier [Google Scholar]
- 22.Sivak J. G., Howland H. C., West J., Weerheim J. 1989. The eye of the hooded seal, Cystophora cristata, in air and water. J. Comp. Physiol. A 165, 771–777 10.1007/BF00610875 (doi:10.1007/BF00610875) [DOI] [PubMed] [Google Scholar]
- 23.Sivak J. G., Lincer J. L., Bobbier W. 1977. Amphibious visual optics of the eyes of the double crested cormorant (Phalacrocorax auritus) and the brown pelican (Pelecanus occidentalis). Can. J. Zool. 55, 782–788 10.1139/z77-102 (doi:10.1139/z77-102) [DOI] [PubMed] [Google Scholar]
- 24.Levy B., Sivak J. G. 1980. Mechanisms of accommodation in the bird eye. J. Comp. Physiol. A 137, 267–272 10.1007/BF00657122 (doi:10.1007/BF00657122) [DOI] [Google Scholar]
- 25.M'Clymont J. R. 1903. How gannets dive. Emu 3, 56 10.1071/MU903055d (doi:10.1071/MU903055d) [DOI] [Google Scholar]
- 26.Oliver W. R. B. 1955. New Zealand birds, 2nd edn Wellington, New Zealand: Reed [Google Scholar]
- 27.Wodzicki K., Robertson F. 1955. Observations on diving of Australasian gannet. Notornis 6, 72–76 [Google Scholar]
- 28.Garthe S., Benvenuti S., Montevecchi W. A. 2000. Pursuit plunging by northern gannets (Sula bassana) feeding on capelin (Mallotus villosus). Proc. R. Soc. Lond. B 267, 1717–1722 10.1098/rspb.2000.1200 (doi:10.1098/rspb.2000.1200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machovsky-Capuska G. E., Vaughn R. L., Würsig B., Katzir G., Raubenheimer D. 2011. Dive strategies and foraging effort in the Australasian gannet Morus serrator revealed by underwater videography. Mar. Ecol. Prog. Ser. 442, 255–261 10.3354/meps09458 (doi:10.3354/meps09458) [DOI] [Google Scholar]
- 30.McGillivray J. 1853. A history of British birds, indigenous and migratory: including their organization, habits and relations. North Br. Rev. 19, 1–24 [Google Scholar]
- 31.Ropert-Coudert Y., Grémillet D., Kato A., Ryan P., Naito Y., Le Maho Y. 2004. A fine-scale time budget of Cape gannets provides insights into the foraging strategies of coastal seabirds. Anim. Behav. 67, 985–992 10.1016/j.anbehav.2003.09.010 (doi:10.1016/j.anbehav.2003.09.010) [DOI] [Google Scholar]
- 32.Ropert-Coudert Y., Daunt F., Kato A., Ryan P. G., Lewis S., Kobayashi K., Mori Y., Grémillet D., Wanless S. 2009. Underwater wingbeats extend depth and duration of plunge dives in northern gannets Morus bassanus. J. Avian. Biol. 40, 380–387 10.1111/j.1600-048X.2008.04592.x (doi:10.1111/j.1600-048X.2008.04592.x) [DOI] [Google Scholar]
- 33.McGillivray J. 1842. Account of the Island of St Kilda, chiefly with reference to its Natural History. Edinburgh New Philos. J. 32, 47–70 [Google Scholar]
- 34.Cunningham R. O. 1866. On the solan goose, or gannet (Sula Bassana, Lim.). Ibis 1, 1–22 10.1111/j.1474-919X.1866.tb06070.x (doi:10.1111/j.1474-919X.1866.tb06070.x) [DOI] [Google Scholar]
- 35.Lee D. N., Reddish P. E. 1981. Plummeting gannets: a paradigm of ecological optics. Nature 293, 293–294 10.1038/293293a0 (doi:10.1038/293293a0) [DOI] [Google Scholar]
- 36.Eriksson M. O. G. 1985. Prey detectability for fish eating birds in relation to fish density and water turbidity. Ornis Scand. 16, 1–7 10.2307/3676567 (doi:10.2307/3676567) [DOI] [Google Scholar]
- 37.Strod T., Izhaki I., Arad Z., Katzir G. 2004. Cormorants keep their power: visual resolution in a pursuit-diving bird under amphibious and turbid conditions. Curr. Biol. 14, 376–377 10.1016/S0960-9822(03)00290-2 (doi:10.1016/S0960-9822(03)00290-2) [DOI] [PubMed] [Google Scholar]
- 38.Strod T., Izhaki I., Arad Z., Katzir G. 2008. Prey detection by great cormorant (Phalacrocorax carbo sinensis), in clear and in turbid water. J. Exp. Biol. 211, 866–872 10.1242/jeb.014324 (doi:10.1242/jeb.014324) [DOI] [PubMed] [Google Scholar]
- 39.White C. R., Day N., Butler P. J., Martin G. R. 2007. Vision and foraging in cormorants: more like herons than hawks? PLoS ONE 2, e 639 10.1371/journal.pone.0000639 (doi:10.1371/journal.pone.0000639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin G. R., White C. R., Butler P. J. 2008. Vision and the foraging technique of Great Cormorants Phalacrocorax carbo: pursuit or close-quarter foraging? Ibis 150, 485–494 10.1111/j.1474-919X.2008.00808.x (doi:10.1111/j.1474-919X.2008.00808.x) [DOI] [Google Scholar]
- 41.Howland H. C., Sayles N. 1985. Photokeratometric and photorefractive measurements of astigmatism in infant and young children. Vis. Res. 25, 73–81 10.1016/0042-6989(85)90082-3 (doi:10.1016/0042-6989(85)90082-3) [DOI] [PubMed] [Google Scholar]
- 42.Borish I. M. 1995. Clinical refraction, 3rd edn Chicago, IL: Professional Press [Google Scholar]
- 43.Schaeffel F., Farkas L., Howland H. C. 1987. Infrared photoretinoscope. Appl. Opt. 26, 1505–1509 10.1364/AO (doi:10.1364/AO) [DOI] [PubMed] [Google Scholar]
- 44.Kröger R. H. H. 2008. The physics of light in air and water. In Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates (eds Thewissen J. G. M., Nummela S.), pp. 113–120 Berkeley, CA: University of California Press [Google Scholar]
- 45.Johnston D. W. 1989. Feeding ecology of pied kingfishers on lake Malawi, Africa. Biotropica 21, 275–277 10.2307/2388655 (doi:10.2307/2388655) [DOI] [Google Scholar]
- 46.Batchelor G. K. 1990. Introduction to fluid dynamics. Cambridge, UK: Cambridge University Press [Google Scholar]
- 47.Cummings M. E., Johnsen S. 2007. Light in the Rocky Shores. In Encyclopedia of tide pools (eds Denny M., Gaines S.), pp. 325–329 Berkeley, CA: University of California Press [Google Scholar]
- 48.Losilevskii G., Weihs D. 2008. Speed limits on swimming of fishes and cetaceans. J. R. Soc. Interface 5, 329–338 10.1017/S0022112084001373 (doi:10.1017/S0022112084001373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machovsky-Capuska G. E., Dwyer S. L., Alley M. R., Stockin K. A., Raubenheimer D. 2011. Evidence for fatal collisions and kleptoparasitism while plunge diving in Gannets. Ibis 153, 631–635 10.1111/j.1474-919X.2011.01129.x (doi:10.1111/j.1474-919X.2011.01129.x) [DOI] [Google Scholar]
- 50.Martin G. R. 1994. Form and function in the optical structure of bird eye. In Perception and motor control in birds (eds Davies M. N. O., Green P. R.), pp. 5–34 Berlin, Germany: Springer [Google Scholar]
- 51.Goldsmith T. H. 1990. Optimization, constraint, and history in the evolution of eyes. Q. Rev. Biol. 65, 281–322 10.1086/416840 (doi:10.1086/416840) [DOI] [PubMed] [Google Scholar]