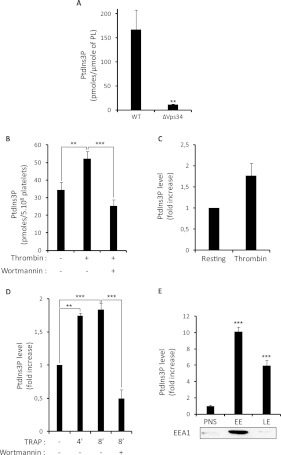
Figure 3. Application of the PtdIns3P mass assay to yeast and mammalian cells.
(A) The amount of PtdIns3P was quantified in wild-type (WT) or vps34-deleted S. cerevisae (ΔVps34) using the mass assay. The amount of PtdIns3P found is expressed as pmole/μmole of total phospholipids (PL) determined by lipid phosphorus assay. Results are the means±S.E.M. from three independent experiments. **P<0.01 according to Student's t test. (B) The amount of PtdIns3P was quantified in resting mouse platelets and in platelets stimulated by 0.5 unit/ml thrombin and pre-incubated or not with wortmannin (100 nM for 15 min). Results are expressed as pmole of PtdIns3P in 5×108 platelets and are the means±S.E.M. from six independent experiments. (C) 32P-labelled mouse platelets were stimulated by 0.5 unit/ml thrombin for 3 min and the level of [32P]PtdIns3P was quantified by HPLC. Results are expressed as the fold increase compared to the level measured in resting platelets, and are the means±S.E.M. of two independent experiments. (D) The amount of PtdIns3P was quantified from human platelets stimulated by 50 μM TRAP for the indicated times (4 or 8 min) in the presence of fibrinogen, with or without wortmannin pretreatment (100 nM for 15 min). Results are expressed as fold increase and are means±S.E.M. of three independent experiments (three different donors). (E) PtdIns3P was quantified in the post-nuclear fraction (PNS) of BHK cells and in isolated early (EE) and late endosomal (LE) fractions. Results are expressed as fold increase and are the means±S.E.M. from three independent experiments. As a control of fractionation, a representative Western blot shows the localization of the early endosome protein EEA1. **P<0.01 and ***P<0.001 determined by one-way ANOVA test.