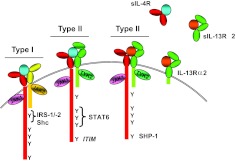
Figure 1. Alternative use of receptor complexes by IL-4 and IL-13.
The IL-4R is composed of two transmembrane proteins. IL-4Rα chain (red) binds IL-4 (blue) with high affinity, leading to dimerization with γc (yellow) or IL-13Rα1 (green), forming the type I or II receptor complex, respectively. IL-13 (orange) binds to IL-13Rα1 with lower affinity, followed by heterodimerization with IL-4Rα to form a high-affinity complex. IL-13 also binds to IL-13Rα2 at the cell surface or in soluble form, but this interaction fails to activate the JAK/STAT pathway, and generally, it is thought to be inhibitory. Soluble (s)IL-4Rα, sIL-13Rα1, and IL-13Rα2 exist and can also bind ligand. Following ligand binding and heterodimerization, JAKs are activated. Tyrosine residues in the cytoplasmic tail of the IL-4Rα become phosphorylated and act as docking sites for signaling molecules. The first cytoplasmic tyrosine residue (Y1) is in a sequence motif called the I4R motif, which interacts with the PTB domains of IRS1 or IRS2. Tyrosine residues 2–4 in the IL-4Rα interact with the SH2 domain of STAT6. The fifth cytoplasmic Y in the IL-4Rα lies in a consensus motif, termed an ITIM that can recruit SHP-1 to the activated receptor complex.