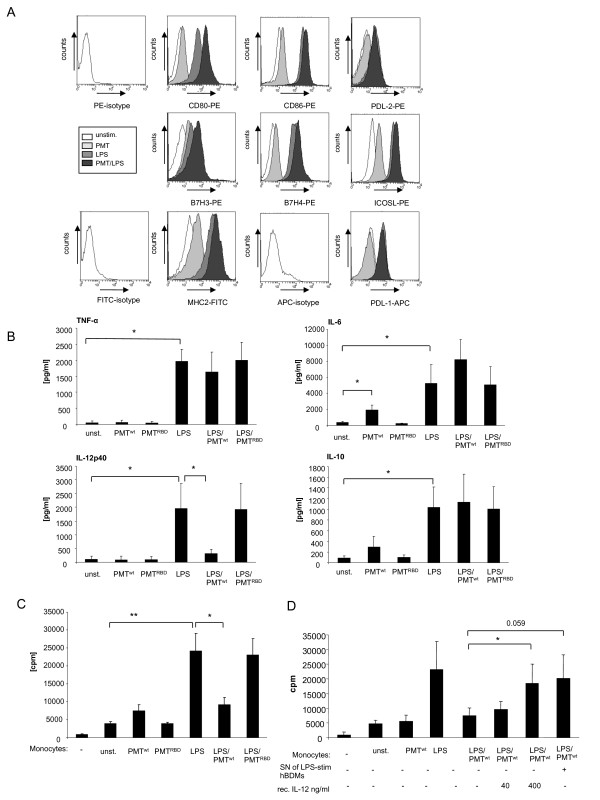
Figure 1.
PMT-mediated modulation of LPS-activated monocytes. A. Monocytes were stimulated with PMTwt (1 μg/ml, 3 h prestimulation) and/or LPS (30 ng/ml; over night stimulation) or left unstimulated. The expression levels of surface markers were analysed by flow cytometry using fluorescently labelled antibodies against CD80, CD86, B7H3, B7H4, ICOSL, MHC2, PDL-1 and PDL-2. The results are presented as overlays which were produced using Weasel v2.5 software. Shown is one representative result from three independent experiments. B. Cells were stimulated as described in A. As additional control inactive PMTRBD (1 μg/ml, 3 h prestimulation) was used. The release of TNF-α, IL-6, IL-10 and IL-12p40 was measured by ELISA. Shown is the mean of three independent experiments (mean ± SD; n = 3) C. Monocytes were stimulated as described in A, washed with media and used for MLR with 1x104 CD14+ hBDMs and 1x105 CD3+ alloreactive T cells. After three days of co-culture proliferation of T cells was analysed by [3H]-thymidine incorporation and presented as counts per minute (cpm). D. Cells were stimulated and T cell proliferation was analysed as described in C. To rescue the T cell-activating ability of LPS/PMT-treated monocytes, co-cultures were supplemented with supernatant (Sn) of LPS-treated monocytes or recombinant IL-12. MLRs were performed in triplicates. Shown is the mean of three independent experiments (mean ± SD; n = 3). Statistical significance was assessed by using student’s t test with *P < 0.05.