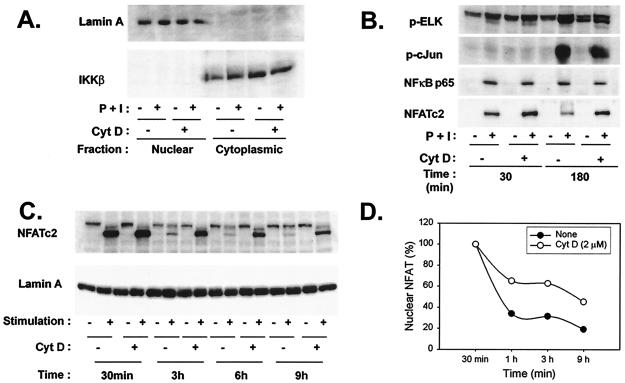
FIG. 5.
Disruption of the actin cytoskeleton results in prolonged NFAT nuclear duration. (A) Separation of nuclear and cytoplasmic fractions is not affected by stimulation or cytochalasin D (CytD) treatment. Th1 cells incubated with culture medium or cytochalasin D (2 μM) for 1 h at 37°C were left either unstimulated or stimulated with P+I for the indicated periods. Cells were then lysed, and nuclear and cytoplasmic fractions obtained. Fractions were analyzed by Western blotting for the presence of the indicated proteins. IKKβ, IκB kinase β. (B) Cytochalasin D treatment results in increased nuclear localization of NFAT but not of other transcription factors relevant to IL-2 production. Th1 T-cell clones were incubated with culture medium or cytochalasin D and were then stimulated with P+I for the indicated times. Nuclear lysates were obtained and analyzed for the presence of the indicated transcription factors by Western blotting. (C) NFAT nuclear localization is prolonged by cytochalasin D. Th1 cells were incubated with culture medium or cytochalasin D, as described above, and were then stimulated with P+I for the indicated times. Presence of NFATc2 in the nucleus was determined by Western blotting of nuclear lysates. The blot was then stripped and reprobed for lamin A as an equivalent loading control. (D) The dephosphorylated NFAT bands shown in panel C were quantified by densitometry analysis. NFAT levels in both untreated and cytochalasin D-treated samples at 30 min were assigned a starting value of 100%, the intensities of all bands were compared to these values, and the percentage of nuclear NFAT in both sets of samples was calculated accordingly.