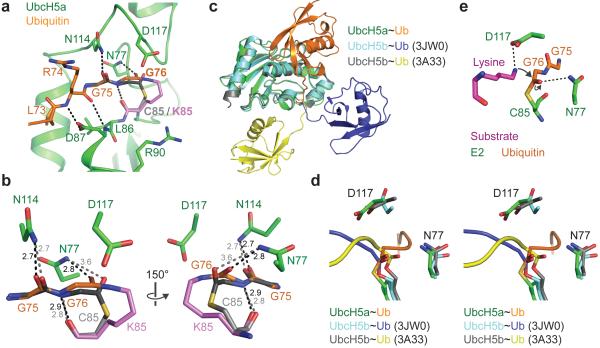
Figure 5. E3-mediated structural changes associated with the catalytically primed form of UbcH5a~Ub.
a, Model of UbcH5a~ubiquitin thioester (grey) with UbcH5a K85 (violet). b, Comparison of modeled thioester with isopeptide linkage. Hydrogen bonds are black (isopeptide) or grey (modeled thioester) dashes. c, Comparison of position of ubiquitin relative to E2 in UbcH5a~ubiquitin–RING complex reported here with UbcH5b~ubiquitin–HECT(NEDD4L) complex (PDB 3JW0)19, and UbcH5b~Ub oxyester (PDB 3A33)18. d, RING mediated remodeling of UbcH5a active site. The position of the C-terminus of ubiquitin linked to the active site cysteine/serine of the E2 is shown relative to residues N77 and D117 in the three structures shown in c. e, Model for nucleophilic attack by substrate lysine (pink) on the E2~Ub thioester bond, based on the SUMO-RanGAP1–Ubc9–RanBP2 structure25.