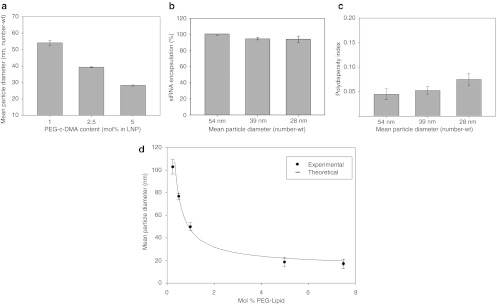
Figure 3.
Increasing PEG-c-DMA content produces progressively smaller lipid nanoparticle (LNP) small interfering RNA (siRNA) systems. (a) Influence of PEG-c-DMA content on LNP size as produced under rapid mixing conditions (4 ml/min total flow rate with an siRNA-buffer:lipid–ethanol volumetric flow rate ratio of 3:1). LNP were composed of DLinKC2-DMA/DSPC/cholesterol/PEG-c-DMA at mol ratios of 40:11.5:47.5:1, 40:11.5:46:2.5, and 40:11.5:43.5:5 for the 1, 2.5, and 5 mol% PEG-c-DMA, respectively. LNP were produced with an siRNA-total lipid ratio of 0.06 wt/wt. (b) Encapsulation efficiency as LNP size is decreased from 42 to 26 nm by increasing the PEG-c-DMA content from 1 to 5 mol%. LNP samples were dialyzed against phosphate-buffered saline (PBS) before measurement of encapsulation. Encapsulation refers to the percentage siRNA present in the LNP following removal of free siRNA using an anionic exchange spin column. (c) Polydispersity of LNP as the size was reduced from 54 to 28 nm by increasing the PEG-c-DMA content from 1 to 5 mol%. The polydispersity index (PDI) was determined as described in legend to Figure 2. (d) Size of empty LNP as a function of PEG-lipid content, which was varied from 0.25–5 mol%. LNP were composed of DLinKC2-DMA/DSPC/cholesterol/PEG-c-DMA, with DLinKC2-DMA and DSPC maintained at 40 and 11.5 mol%, respectively. Titration of PEG-c-DMA was compensated by adjustment of cholesterol. All LNP were produced at an initial lipid concentration of 20 mmol/l in the lipid-ethanol phase prior to mixing with 25 mmol/l acetate buffer, pH 4. Number-weighted mean diameters are shown for the LNP following dialysis against PBS to remove residual ethanol and increase the pH to 7.4. Error bars represent standard deviation from mean (n = 3). DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine.