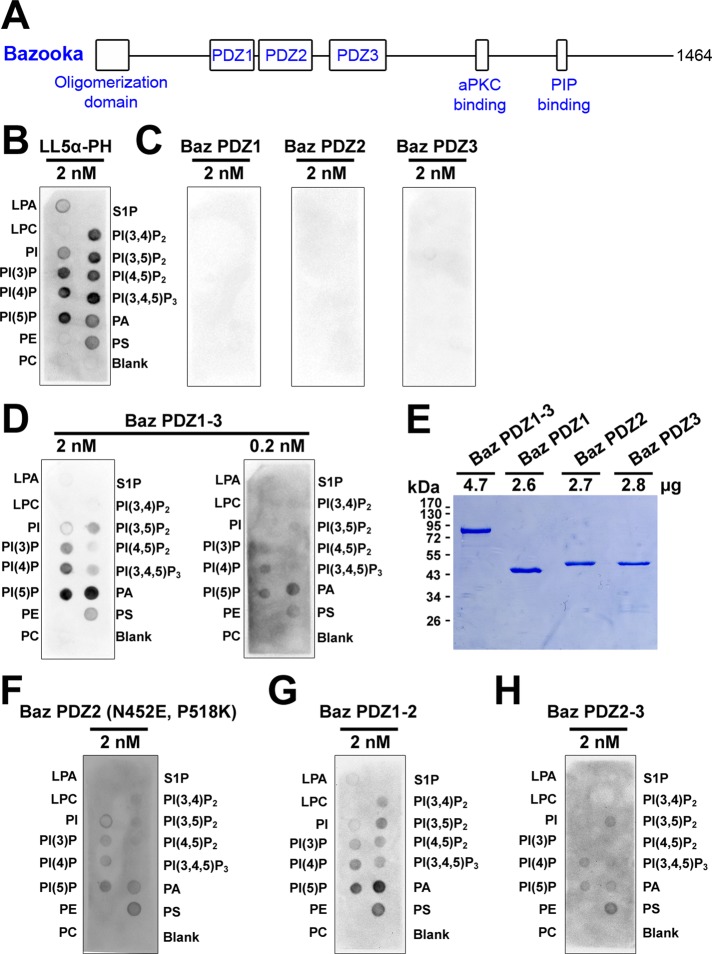
FIGURE 1:
Preferential binding of tandem Bazooka PDZ domains to immobilized phosphatidic acid. (A) A schematic of Baz. (B–D, F–H) GST antibody detection of GST-fusion proteins bound to immobilized lipids: lysophosphatidic acid (LPA), lysophosphocholine (LPC), phosphatidylinositol (PI), phosphatidylinositol (3) phosphate (PI(3)P), phosphatidylinositol (4) phosphate (PI(4)P), phosphatidylinositol (5) phosphate (PI(5)P), phosphatidylethanolamine (PE), phosphatidylcholine (PC), sphingosine 1-phosphate (S1P), phosphatidylinositol (3,4) bisphosphate (PI(3,4)P2), phosphatidylinositol (3,5) bisphosphate (PI(3,5)P2), phosphatidylinositol (4,5) bisphosphate (PI(4,5)P2), phosphatidylinositol (3,4,5) trisphosphate (PI(3,4,5)P3), phosphatidic acid (PA), phosphatidylserine (PS). (B) The LL5α-PH domain (MultiPIP Grip) preferentially binds phosphoinositides. (C) Individual Baz PDZ domains show no detectable lipid binding. (D) Baz PDZ1-3 preferentially binds PA over a 10-fold concentration range. (E) 10% SDS–PAGE and staining with Coomassie brilliant blue shows that the PDZ proteins used in C and D had similar concentrations and stabilities. (F) Converting amino acid residues in Baz PDZ2 to those responsible to phosphoinositide binding in Rat PDZ2 (Wu et al., 2007) conveys lipid binding activity. (G) Baz PDZ1-2 preferentially binds PA. (H) Baz PDZ2-3 also binds lipids but with a reduced preference for PA.