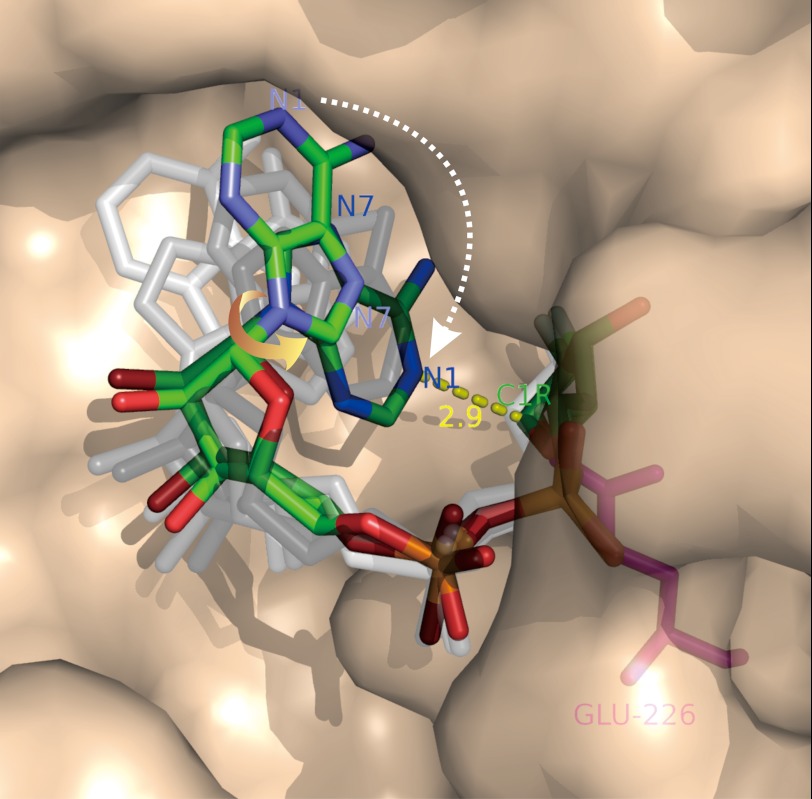
FIGURE 3.
Imaging the NAD cyclization by CD38. A mutant of CD38 with Glu-146 changed to alanine (described in text) was co-crystallized with a close analog of NAD, 2′-deoxy-2′-fluoroarabinoside NAD, which forms a covalent linkage between its ribosyl anomeric carbon (C1R) and the catalytic residue Glu-226. Six molecules of the complex are found in each of the asymmetric crystal units, each containing a covalent intermediate in a different conformation. The conformations relate to each other by single-bond rotation, indicating that they represent substates of the cyclization process. Ordering the conformations shows that the cyclization requires a 180° rotation of the adenine ring around its glycosidic bond (yellow arrow), such that the N1 of the adenine is put into close proximity to the ribosyl anomeric carbon (2.9 Å; yellow dashed line), the site of cyclization. Rendered in colored sticks are the starting state, where the adenine ring is farthest from the ribosyl anomeric carbon, and the ending state, where the two are closest. The substates representing various degrees of single-bond rotation of the intermediate between the starting and ending states are colored in shades of gray. Red, oxygen; orange, phosphorus; green, carbon; blue, nitrogen.