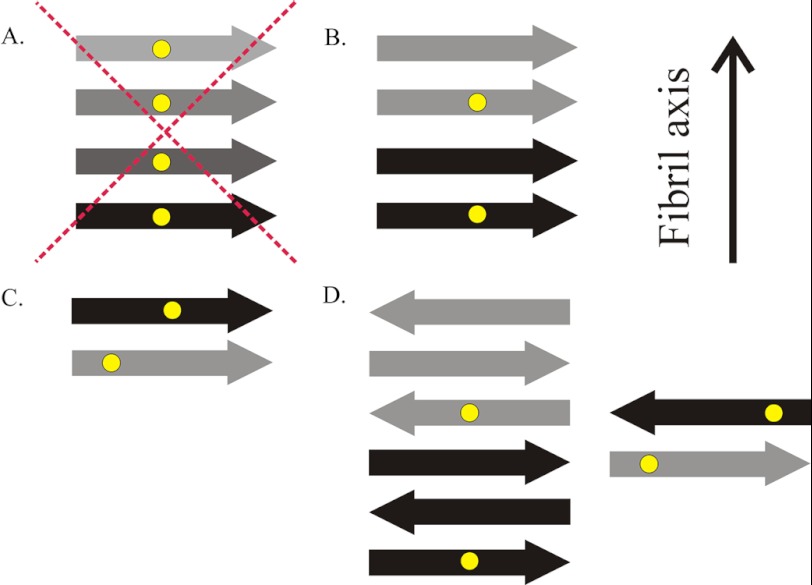
FIGURE 6.
Sketches of different types of cross-β structures. A, HDx1 does not adopt a parallel, in-register structure common to other disease-causing amyloid proteins, in which multiple spin labels (yellow dots) come into close contact. B, one potential structure in which spin labels approach within 20 Å is a parallel arrangement of β-strands (arrows) in which each molecule takes up more than one layer and spin labels are separated by one or more intervening layers of β-strands. C, another possibility would be a parallel structure in which each molecule takes up a separate layer, but, due to the promiscuous nature of polyQ, labels are not in register. D, alternatively, several antiparallel models are possible, with or without intervening β-strands. The fibril axis is indicated, which is also the direction of main chain hydrogen bonds. Different shades of arrows represent β-strands from different HDx1 molecules.