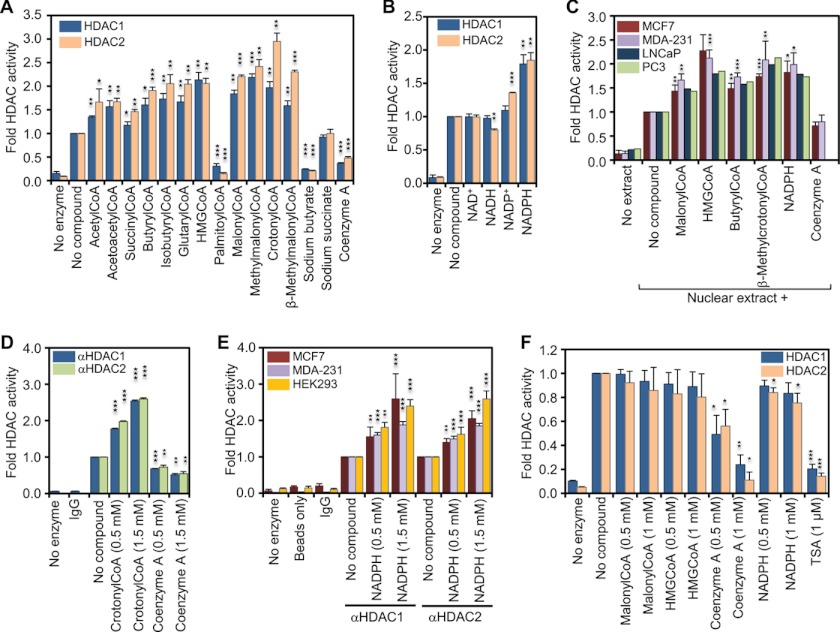
FIGURE 1.
Coenzyme A derivatives and NADPH increase the in vitro activity of HDAC1 and HDAC2. A, recombinant HDAC1 and -2 were incubated with saturating amounts (50 μm) of 3H-labeled histones in the absence or presence of the indicated metabolites (1 mm). Released 3H-labeled acetate was extracted and measured by scintillation counting. The graph represents the fold change in HDAC activity compared with basal activity. B, same as in A but in the absence or presence of 1 mm of the indicated nicotinamide dinucleotides. C, in vitro HDAC activity of 1 μg of nuclear extract from each of human breast cancer cell lines MCF7 and MDA-MB-231 and the prostate cancer cell lines LNCaP and PC3 was determined and reported as in A. D, HDAC1- and HDAC2-complexes were immunoprecipitated from MDA-MB-231 whole cell extract, and HDAC activity was assessed in the presence or absence of 0.5 and 1.5 mm of either crotonyl-CoA or free CoA. Immunoprecipitation with nonspecific rabbit IgG and no enzyme controls was used to determine assay background. E, immunoprecipitated HDAC1 and -2 complexes from MCF7, MDA-MB-231, and the embryonic kidney cell line HEK293 were used to assess the in vitro activity in the presence and absence of NADPH. Immunoprecipitation with nonspecific rabbit IgGs, no- enzyme and a protein A-Dynabeads only controls were used to determine background levels. F, recombinant HDAC1 and -2 were incubated with saturating amounts of acetylated substrate Fluor de Lys in the absence or presence of the indicated metabolites. HDAC activity was determined by fluorimetry. The graph represents the fold change in HDAC activity compared with basal activity in absence of metabolites. Error bars indicate standard deviation of three independent experiments. A two-tailed Student's t test was used to calculate p values (*, p < 0.05; **, p < 0.01; ***, p < 0.001).