Background: ABCB6 is an ATP binding cassette transporter that regulates heme biosynthesis.
Results: Polyaromatic hydrocarbons increase heme synthesis in liver by activating ABCB6 expression via the aryl hydrocarbon receptor.
Conclusion: ABCB6 is required for PAH-mediated induction of heme biosynthesis.
Significance: ABCB6 expression might be clinically relevant in polyaromatic hydrocarbon-induced porphyrias and carcinogenesis.
Keywords: ABC Transporter, Aryl Hydrocarbon Receptor, Gene Regulation, Heme, Promoters, Polyaromatic Hydrocarbon
Abstract
Liver is endowed with a mechanism to induce hepatic cytochromes P450 (CYP450s) in response to therapeutic drugs and environmental contaminants, leading to increased detoxification and elimination of the xenobiotics. Each CYP450 is composed of an apoprotein moiety and a heme prosthetic group, which is required for CYP450 activity. Thus, under conditions of CYP450 induction, there is a coordinate increase in heme biosynthesis to compensate for the increased expression of CYP450s. ABCB6, a mitochondrial ATP binding cassette transporter, which regulates coproporphyrinogen transport from the cytoplasm into the mitochondria to complete heme biosynthesis, represents a previously unrecognized rate-limiting step in heme biosynthesis. However, it is not known if exposure to drugs and environmental contaminants induces ABCB6 expression, to assure an adequate and apparently coordinated supply of heme for the generation of functional cytochrome holoprotein. In the present study, we demonstrate that polycyclic aromatic hydrocarbons (PAHs), the widely distributed environmental toxicants shown to induce porphyrin accumulation causing hepatic porphyria, up-regulate ABCB6 expression in both mice and humans. Using siRNA technology and Abcb6 knock-out mice, we demonstrate that PAH-mediated increase in hepatic porphyrins is compromised in the absence of ABCB6. Moreover, in vivo studies in aryl hydrocarbon receptor (AhR) knock-out mice demonstrate that PAH induction of ABCB6 is mediated by AhR. Promoter activation studies combined with electrophoretic mobility shift assay and chromatin immunoprecipitation assay demonstrate direct interactions between the AhR binding sites in the ABCB6 promoter and the AhR receptor, implicating drug activation mechanisms for ABCB6 similar to those found in inducible cytochrome P450s. These studies are the first to describe direct transcriptional activation of both mouse and human ABCB6 by xenobiotics.
Introduction
Heme is indispensable for mammalian life. It is an essential component of numerous heme proteins, with functions including oxygen transport, energy metabolism, and drug biotransformation (1–3). Under normal physiological conditions, intracellular free heme levels are extremely low because increased levels of free heme are cytotoxic, and accordingly heme biosynthesis is tightly regulated (4–6). However, the rate of heme biosynthesis must also be responsive to increased demands, for instance, during induction of drug-metabolizing cytochromes P450 (CYP450s),2 which is required to assure an adequate and apparently coordinated supply of heme for the generation of functional cytochrome holoprotein (7–10). Under these conditions, heme biosynthesis is swiftly up-regulated to provide sufficient heme to nascent apocytochromes.
The two major sites of heme synthesis are bone marrow, where hemoglobin is produced, and liver, where various hemoproteins (in particular, microsomal CYP450s) rely on prosthetic heme to catalyze the oxidation of endogenous and exogenous compounds. The rate of heme synthesis in both the bone marrow and the liver is controlled at the first committed step, the condensation of glycine and succinyl-CoA to 5-aminolevulinate (11). This committed step is catalyzed by 5-aminolevulinic acid synthase (ALAS), which exists as two isoforms: ALAS1, which regulates heme synthesis in liver and other organ systems, and ALAS2, which regulates heme synthesis in hematopoietic tissues (12–14). The regulatory role of ALAS in heme synthesis is underlined by the fact that ALAS mRNA is markedly increased under physiological conditions demanding more heme, such as exposure to drugs and environmental toxicants, whereas expression levels of the other enzymes in the pathway do not change significantly (15). Recent studies have shown that this increase in ALAS expression in response to heme demand is not a consequence of heme feedback regulation but a direct activation of ALAS transcription by xenobiotic-sensing nuclear receptors similar to those found in inducible cytochrome P450s (16).
Although ALAS-mediated regulation of heme synthesis is considered the key step in heme biosynthesis, recent reports have identified a second regulatory step in heme biosynthesis mediated by the mitochondrial ATP binding cassette transporter ABCB6 (17). ABCB6 is highly expressed in fetal liver, erythroid cells, and adult tissues that have substantial heme requirements because of their high metabolic activity (e.g. heart and skeletal muscle) (17). Further, ABCB6 expression is directly related to enhanced de novo porphyrin biosynthesis, and ABCB6/Abcb6 overexpression activates the expression of genes important for heme biosynthesis (17). Thus, ABCB6 represents a previously unrecognized rate-limiting step in heme biosynthesis. Supporting this hypothesis, recent observations demonstrate that ABCB6 mRNA, like ALAS mRNA, is markedly increased under physiological conditions demanding more heme (17–19). Despite these observations, very little is known about the mechanisms that regulate ABCB6 expression both under normal physiological conditions and under conditions of increasing demand for heme.
In the present study, we demonstrate that exposure to environmental contaminants, such as tetrachlorodibenzo-p-dioxin (TCDD), benzo[a]pyrene (B[a]P), and 3-methylcholanthrene (3-MC), three of the most comprehensively studied prototypes of polycyclic aromatic hydrocarbons, induces ABCB6 expression in a dose- and time-dependent manner in both humans and mice. We show that TCDD- and B[a]P-mediated induction of ABCB6 requires activation of the nuclear receptor AhR, which binds and interacts with AhR-responsive elements in the 5′-flanking region of the gene encoding ABCB6. More importantly, we demonstrate that B[a]P-mediated increase in hepatic porphyrin accumulation is in part dependent on ABCB6 expression.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
Human liver-derived cell lines HepG2 and Huh7 were purchased from the American Type Culture Collection (Manassas, VA). HepG2 and Huh7 cells were cultured in modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 2 mm l-glutamine, and 100 units/ml penicillin. HepG2 and Huh7 cells were engineered to overexpress human ABCB6 as described (17, 20). ABCB6-overexpressing cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 100 units/ml penicillin, 2 mm l-glutamine, and 0.6 μg/ml puromycin. Chemicals were purchased from Sigma-Aldrich unless otherwise indicated. Oligonucleotides, including biotinylated DNA, were synthesized at Integrated DNA Technologies (Coralville, IA). In the dose-dependent studies, cells were treated with 0, 2.5, 5, or 10 μm B[a]P as well as DMSO solvent for 16 h or with 0, 1, or 2 nm TCDD. In-time dependent studies, cells were treated with 10 μm B[a]P or 2 nm TCDD for 0, 2, 4, 8, or 16 h.
Cytotoxicity Assay
Cell viability was measured in logarithmically growing mouse primary hepatocytes and HepG2 and Huh7 cells as described (21). Briefly, cells were plated onto 96-well plates at a starting density of 104 cells and treated with increasing concentrations of TCDD, B[a]P, or the respective solvents. Cell viability was determined at 24, 48, and 72 h using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide. Absorbance at 570 nm was measured with a kinetic microplate reader (BioTek, Winooski, VT) and was used as a measure of cell viability (21).
Real-time PCR and Western Analysis
Real-time PCR was performed as described previously (17) using primer sets specific for the human ABCB6 or mouse Abcb6 gene, human or mouse Alas1, and human or mouse Actin gene. For Western analysis, the mitochondrial fraction was prepared as described below (and in Ref. 18), and 100 μg of mitochondrial protein was analyzed by PAGE. Blots were probed with anti-ABCB6 (20, 21) and anti-porin antibodies (Mitosciences, Eugene, OR). We detected the secondary antibody by using a chemiluminescence detection kit (Amersham Biosciences). ABCB6 antibodies were generated using a portion of the ABCB6 protein (amino acids 592–894) that is predicted to localize to the cytosol and is unique among the ABC transporters. The antibody was affinity-purified and characterized for its ability to recognize the native ABCB6 protein (20, 21).
Isolation and Purification of Mitochondria
Cells were pelleted in Hanks' buffered saline solution (Invitrogen), resuspended in buffer A (10 mmol/liter NaCl, 1.5 mm MgCl2, and 10 mmol/liter Tris (pH 7.4)) containing protease inhibitor mixture (Roche Applied Science), swollen on ice, and disrupted with a type B Dounce homogenizer. Buffer B (525 mmol/liter mannitol, 175 mmol/liter sucrose, 12.5 mmol/liter Tris (pH 7.4), and 2.5 mmol/liter EDTA) was added in a ratio of 4:10 homogenate/buffer B. The supernatant was collected after centrifugation at 1500 × g for 10 min. The supernatant was centrifuged at 17,000 × g for 15 min to pellet mitochondria. The crude mitochondria were purified from the endoplasmic reticulum as described previously (18).
Cellular Protoporphyrin IX Measurement
Intracellular protoporphyrin IX concentration was measured as described previously (17). Briefly, cells were harvested and washed once with PBS. Protoporphyrin IX concentration was measured by using a Vantage flow cytometer (BD Biosciences). To induce protoporphyrin IX fluorescence, the excitation wavelength was set at 405 nm, and the emission filter was set at 695 nm/40 nm.
Primary Mouse Hepatocyte Culture
Hepatocytes from the liver were isolated using the collagenase perfusion method as described previously (22). Briefly, under pentobarbital anesthesia (50 mg/kg intraperitoneally), liver was perfused with 50 ml of calcium- and magnesium-free Hanks' balanced salt solution (Sigma) supplemented with 0.5 mm EGTA, 5.5 mm glucose, and penicillin-streptomycin (Sigma), followed by 40 ml of calcium- and magnesium-free Hanks' balanced salt solution supplemented with 1.5 mm calcium chloride, 5.5 mm glucose, penicillin-streptomycin, and 0.02 g of Type IV collagenase (Sigma). Liver was removed, and the digested product was centrifuged at 50 × g for 2 min to pellet the hepatocytes. The hepatocytes were washed three times with Williams' medium E (Invitrogen) and then cultured in Williams' medium E containing 10% FBS and penicillin-streptomycin. After a 3-h attachment period, the medium with unattached cells was removed, and fresh medium was added. The viability of isolated hepatocytes was >90% by the criterion of trypan blue (Sigma) exclusion. Cells were cultured for ∼16 h before the addition of drugs.
Animals and Treatments
AhR gene-deleted mice (AhR−/−) were a kind gift from Dr. Curtis Klaassen (University of Kansas Medical Center). Abcb6 gene-deleted mice (Abcb6−/−) were developed on a C57BL6 background, using homologous recombination to replace exons 3, 4, and 5 in the Abcb6 gene with the neomycin resistance cassette as described.3 Wild-type mice were C57BL6 from the Jackson Laboratory (Fig. S4). Mice were housed in polycarbonate cages (4 mice/cage), provided a normal diet and water ad libitum, and maintained on a 12/12-h light/dark cycle at 22 ± 5 °C and 50 ± 20% relative humidity. At 8 weeks of age, AhR+/+ and AhR−/− (4 mice/group/experiment) were given 37 μg/kg TCDD or an equal volume of vehicle (corn oil) intraperitoneally for 4 days. Animals were sacrificed at the end of treatment. RNA was isolated from livers of mice in TRIzol (Invitrogen), and complementary DNA generated from the RNA was used for real-time PCR as described above.
The effect of TCDD on Abcb6 expression was measured in 8-week-old C57BL6 mice. Mice (6 mice/group) were given TCDD (37 μg/kg in corn oil) intraperitoneally for 4 days. All mice survived TCDD treatment. At the end of the treatment, mice were sacrificed, liver was harvested, and RNA and mitochondria were isolated immediately as described above and used in real-time PCR or immunoblots.
ABCB6/Abcb6 Promoter Analysis
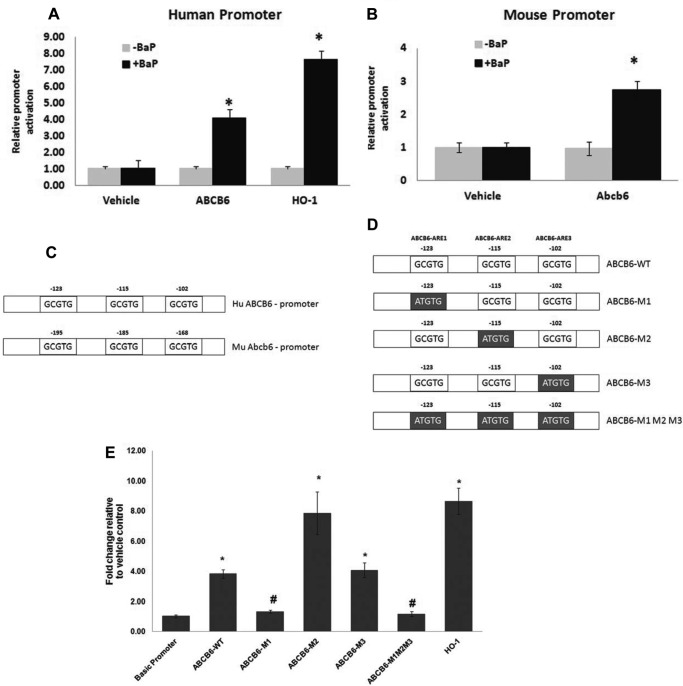
Promoter analysis was performed as described previously (23). Briefly, HepG2 cells at ∼60% confluence were transfected with 1.5 μg/well of either pGL2-luciferase, pLightSwitch-ABCB6-luciferase (Switchgear Genomics, Menlo Park, CA), pGL2-Abcb6-luciferase, or pGL2-HO-1-luciferase constructs using Lipofectamine reagent following the manufacturer's protocol (Invitrogen). All transfections included Renilla luciferase (100 ng/well) as an internal transfection control. In a subset of experiments, we introduced aryl hydrocarbon-responsive element (AhRE) mutations into the ABCB6 promoter by using a site-directed mutagenesis kit (Stratagene, Santa Clara, CA). Briefly, two nucleotide mutations (consensus motifs 5′-GCGTG-3′ mutated to 5′-ATGTG-3′) were introduced into the AhR binding site of the ABCB6 promoter at base pairs −102 bp, −115 bp, and −123 bp relative to the transcription start site (see Fig. 6D). All mutations were confirmed by sequencing using pGL2-basic and pLightSwitch-ABCB6-luciferase primers (Promega, Madison, WI). The relative activity of the promoter constructs was determined after subtraction of the values obtained for pGL2-luciferase, and the results were expressed in relative terms (ratio of vehicle control value to treatment value). All experiments were performed at least three times with a minimum of four replicates per experiment.
FIGURE 6.
Active AhR response element in human and mouse 5′-flanking region. Shown is activity of human (A) and mouse (B) 5′-flanking sequence in the Abcb6 gene in response to B[a]P. C, schematic representation of the 5′-flanking region of human and mouse Abcb6 genes. Core AhREs are shown as boxes with respective locations of the 5′ end base from the reported transcription start sites. D, schematic representation of the human ABCB6 promoter, used in the transactivation studies, showing the introduction of mutations in the AhRE. Gray shaded boxes show the AhR sequence motif that was mutated by site-directed mutagenesis on the human ABCB6 promoter. E, mutation of either the distal AhRE or all of the three AhREs does not activate the promoter-luciferase reporter in response to B[a]P. Values represent mean ± S.D. (error bars) (n = 3). Results representative of six independent experiments. *, significantly different from empty vector-transfected cells treated with B[a]P and ABCB6 promoter-transfected cells treated with vehicle. p < 0.01. #, significantly different from ABCB6-WT, ABCB6-M1, and ABCB6-M2 promoter-transfected cells treated with B[a]P. p < 0.01.
Electrophoretic Mobility Shift Assay (EMSA)
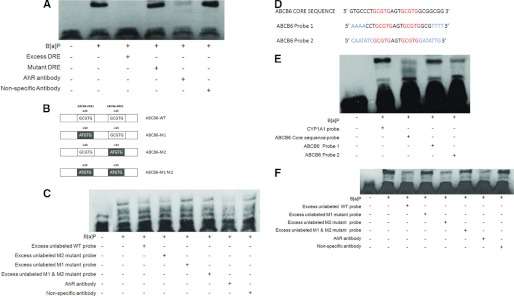
Electrophoretic mobility shift assay was performed as described previously (23). Briefly, HepG2 cells at ∼90% confluence were treated with vehicle or 5 μm B[a]P for 2 h, and nuclear protein was extracted using the NE-PER nuclear extraction kit (Pierce). To detect AhRE interaction, 20 fmol of biotinylated DNA segments containing either 1) the three AhREs of human ABCB6 (CGTACGTGCCCTGCGTGAGTGCGTGGCGGCGGCGCGTGCG; the core AhRE is underlined (Fig. 6D)), 2) only the two distal AhREs (CGTACGTGCCCTGCGTGAGTGCGTGGCGGCGG; underlined and in boldface type (Figs. 6d and 7D)), or 3) the two distal AhREs where the sequences flanking the core AhRE were changed to random bases (Fig. 7D) were incubated with 2 μg of nuclear extracts with or without excess amounts (4 pmol) of similar or mutant (CGTACGTGCCCTATGTGAGTATGTGGCGGCGGCATGTG; mutations shown in italic type) unbiotinylated DNA oligonucleotides. Rabbit polyclonal antibody against AhR (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used to probe the AhRE complexes, and anti-FXR polyclonal antibody (Santa Cruz Biotechnology, Inc.) was used as a nonspecific negative control in the experiment. A 25-bp biotinylated DNA motif containing human CYP1A1 (cytochrome P450 subfamily 1A1) AhRE (5′-CGAGTTGCGTGAGAAGAGCCAGATC- 3′; the core AhRE is underlined) and the mutant CYP1A1 AhRE (5′-CGAGTTGATTGAGAAGAGCCAGATC- 3′; mutations shown in italic type) was used as a positive control in these experiments.
FIGURE 7.
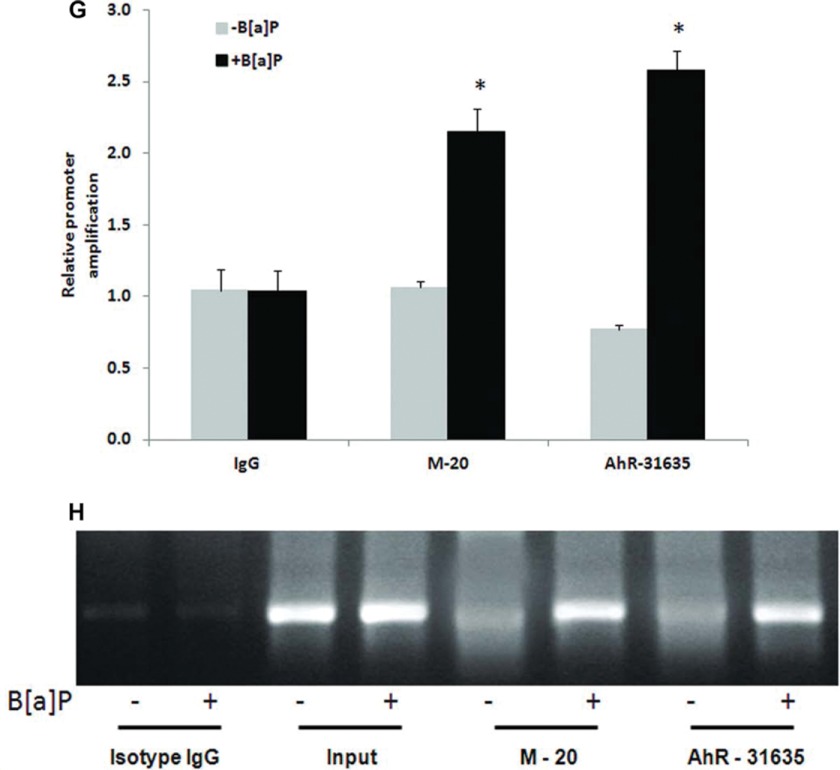
AhR is recruited to the distal AhR response element in the human ABCB6 promoter. A, electrophoretic mobility shift assay of AhR complex binding to the AhRE element in the CYP1A1 promoter. B, schematic representation of the human ABCB6 promoter used in EMSA studies. C, electrophoretic mobility shift assay of AhR complex binding to the AhRE element in the human ABCB6 promoter. D, schematic representation of the changes introduced in the AhRE flanking sequence. Letters highlighted in blue show the altered bases introduced in the AhRE flanking sequence, and letters in red show the core AhRE. E and F, electrophoretic mobility shift assay of AhR complex binding to the human ABCB6-WT promoter and to the human ABCB6 promoter carrying changes to the AhRE flanking sequence. In all EMSA assays (A, C, E, and F), biotinylated DNA probes containing AhRE were incubated with nuclear extracts of HepG2 cells treated with either vehicle or 5 μm B[a]P in the presence or absence of excess unlabeled probe or the mutant AhREs. Polyclonal antibody against AhR was used to show antibody-induced reduction in the intensity of the AhR-protein complex band. Incubation with equal amounts of a nonspecific polyclonal antibody did not reduce the band intensity. Results are representative of four independent experiments. G and H, chromatin immunoprecipitation analysis shows recruitment of AhR to the AhRE in the ABCB6 promoter. Histograms (G) represent real-time PCR values of promoter amplification, whereas H shows conventional PCR analysis (28 cycles) for AhREs of ABCB6 to confirm the specificity of PCR amplification and quantitation in SYBR green real-time PCR. Immunoprecipitation was carried out with two anti-AhR antibodies (M-20 and AhR-31635) or isotype control IgG. Values represent mean ± S.D. (error bars) of three independent experiments. *, significantly different from B[a]P-treated isotype IgG control values. p < 0.01.
RNA Interference
ABCB6-shRNA and scrambled shRNA viral particles were obtained from Sigma. Stable cell lines harboring either ABCB6-shRNA or the scrambled shRNA were generated by transduction of viral particles following the manufacturer's protocol, followed by selection in puromycin (0.6 μg/ml for both HepG2 and Huh7 cells) as described (20). Loss of endogenous ABCB6 expression was confirmed by RT-PCR and immunoblot using gene-specific primers and protein-specific antibody.
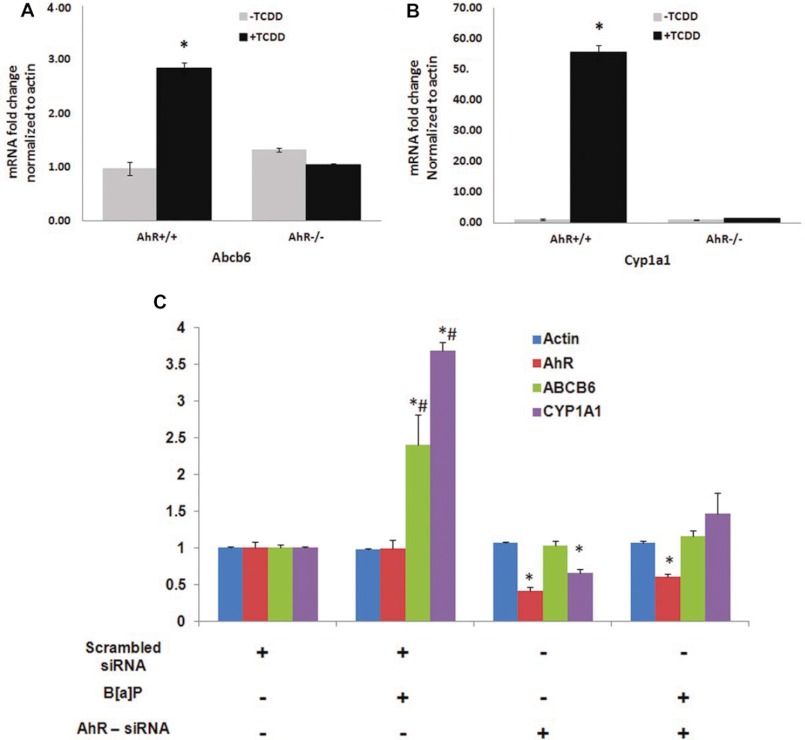
The siRNA specific for AhR and a scrambled siRNA were a kind gift from Dr. Hongbing Wang (University of Maryland, Baltimore, MD) (24). HepG2 cells at ∼60% confluence were transfected with AhR siRNA or scrambled siRNA (40 pmol/well) using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. Twenty-four hours after siRNA transfection, cells were treated with either DMSO or 5 μm B[a]P and incubated for an additional 16 h before harvesting. Total RNA isolated from transfected cells was used for cDNA synthesis, and real-time PCR analysis was performed as described above using gene-specific primers for AhR, CYP1A1, ABCB6, and Actin.
Chromatin Immunoprecipitation (ChIP) Assay
Chromatin immunoprecipitation was performed using HepG2 cells treated with vehicle or B[a]P (5 μm) for 2 h. Approximately 2 × 106 cells were cross-linked with 1% formaldehyde for 15 min at room temperature and washed with ice-cold phosphate-buffered saline containing a protease inhibitor mixture. Chromatin derived from isolated nuclei was fragmented to an average size of 0.5–2 kb using a Vibra-cell ultrasonic processor (Sonics, Newton, CT). Following centrifugation, supernatants containing sheared chromatin were immunoprecipitated overnight at 4 °C with anti-AhR antibody (M-20 from Santa Cruz Biotechnology, Inc., and AhR-31635 from Aviva) or isotype control IgG. The immunocomplex was precipitated using protein A coupled to Sepharose beads and decross-linked for 6 h. The immunoprecipitated DNA fragments were recovered by QIAquick PCR purification kit (Qiagen). Quantitative RT-PCR was performed using a specific set of primers (sense, 5′-CAGAGCCAGCGGGGCCGTGCTG-3′; antisense, 5′-GGCGCGGACATCCGGGTGCC-3′) spanning the region between −20 and −193 bp around the putative AhR binding site within the ABCB6 promoter. PCR products were also resolved on a 1.5% agarose gel and visualized by ethidium bromide staining.
Statistical Analysis
Statistical analysis of the observed values was performed using Student's t test. All calculations were performed with the SPSS statistical software package (SPSS Inc., Chicago, IL). All values are expressed as mean ± S.D. Significant differences between the groups were determined with SPSS 10.0 software (SPSS Inc.). A difference was considered significant at the p < 0.05 level.
RESULTS
Polyaromatic Hydrocarbon (PAH) Increases Hepatic Porphyrin Levels in Mice and Humans
PAHs are potent atmospheric pollutants that are classified as either being nontoxic or being extremely toxic based on their structure (25–27). The three prototypical PAHs used in the studies described in this manuscript, TCDD, B[a]P, and 3-MC are classified as teratogens by the Environmental Protection Agency and are considered to be extremely toxic (28–30). Thus, in these studies, we first established the toxicity profile for TCDD, B[a]P, and 3-MC in mouse primary hepatocytes and human hepatoma cells. Mouse primary hepatocytes and human hepatoma cells (HepG2 and Huh7) were treated with increasing concentrations of either B[a]P (0, 2.5, 5, and 10 μm), TCDD (0, 1, and 2 nm), or 3-MC (0, 5, 10, and 20 μm) for 16 h, and viability was evaluated by a trypan blue exclusion assay (to measure necrosis) and annexin V staining (to measure apoptosis). We found that B[a]P was non-lethal up to a concentration of 10 μm, TCDD was non-lethal up to a concentration of 2 nm, and 3-MC was non-lethal up to a concentration of 20 μm, for a maximum exposure time of 16 h (data not shown). Based on these initial observations, an exposure regimen of 0, 2.5, 5, and 10 μm for 16 h for B[a]P; 0, 1, and 2 nm for 16 h for TCDD; and 0, 5, 10, and 20 μm for 3-MC was considered as non-lethal to both mouse primary hepatocytes and human hepatoma cells and was employed as the treatment regimen in the rest of the studies.
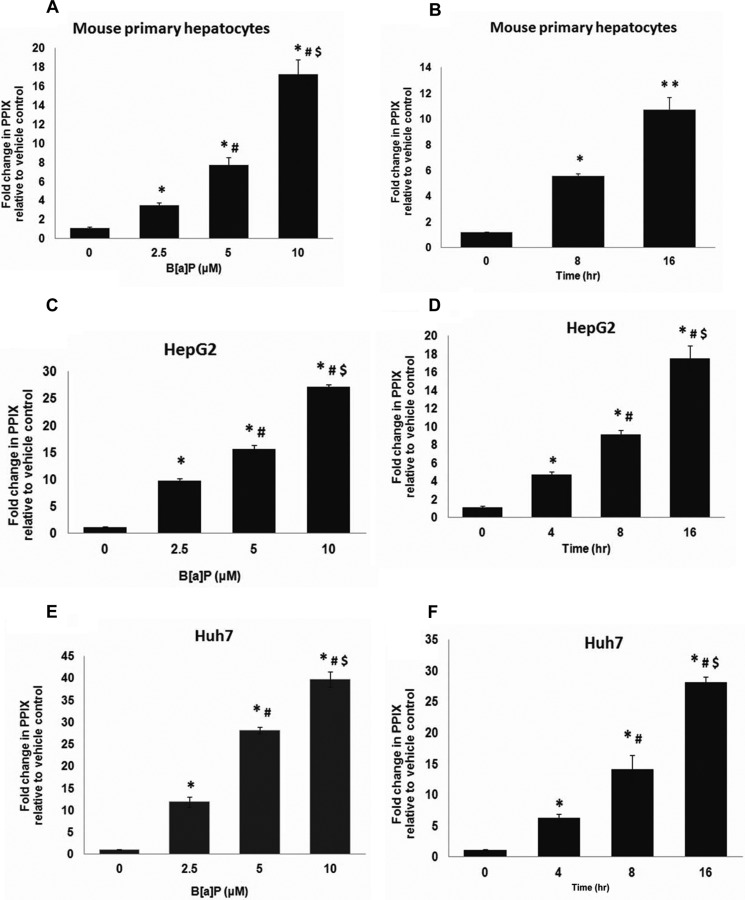
Previous studies have shown that exposure to PAHs, such as B[a]P, 3-MC, and TCDD, induces the expression and activity of CYP450s. This increase in CYP450 expression would require a coordinate increase in cellular porphyrin levels to compensate for the increased heme demand required for increased CYP450 activity (31–34). To test this, we evaluated the effect of B[a]P, 3-MC, and TCDD on hepatic protoporphyrin IX (protoporphyrin IX) levels, as a measure of heme, using the non-lethal concentrations of B[a]P, 3-MC, and TCDD described above. In these studies, both dose- and time-dependent effects of B[a]P, 3-MC, and TCDD on hepatic protoporphyrin IX levels were evaluated. In the dose-dependent studies, mouse primary hepatocytes and human hepatoma cells were exposed to increasing concentrations of either B[a]P (0–10 μm), 3-MC (0–20 μm), or TCDD (0–2 nm) for 16 h. In the time-dependent studies, mouse primary hepatocytes and human hepatoma cells were exposed to a single concentration of either 10 μm B[a]P, 10 μm 3-MC, or 2 nm TCDD for 0, 2, 4, 8, and 16 h. We found that both B[a]P and 3-MC increased cellular protoporphyrin IX levels in a dose-dependent (Fig. 1A and supplemental Fig. 1) and time-dependent manner in both primary hepatocytes (Fig. 1B (B[a]P) and data not shown (3-MC)) and in hepatoma cells (Fig. 1, C–F, and supplemental Fig. 1). Maximum increase in cellular protoporphyrin IX was observed after 16 h of exposure to 10 μm B[a]P or 20 μm 3-MC. The percentage -fold increase in protoporphyrin IX levels in response to B[a]P and 3-MC treatment was comparable between mouse primary hepatocytes and human hepatoma cells, suggesting a similar effect of B[a]P and 3-MC on protoporphyrin levels in both mice and humans. In contrast to B[a]P and 3-MC, TCDD did not increase hepatic protoporphyrin IX levels (data not shown), consistent with previous observations (36).
FIGURE 1.
Polyaromatic hydrocarbon benzo[a]pyrene increases hepatic protoporphyrin IX levels. Exposure to B[a]P increases protoporphyrin IX levels in both mouse primary hepatocytes (A and B) and human hepatomas (C–F), in a dose-dependent (A, C, and E) and time-dependent (B, D, and F) manner. Values represent mean ± S.D. (error bars) (n = 4). Results shown are representative of three independent experiment with n = 4/experiment. *, significantly different from vehicle control (p < 0.01); #, significantly different from cells treated with 2.5 μm B[a]P in dose-dependent studies and significantly different from 4-h treatment (hepatomas) in time-dependent studies (p < 0.01). $, significantly different from cells treated with 5 μm B[a]P in dose-dependent studies and significantly different from 8-h treatment (hepatomas) in time-dependent studies (p < 0.01). **, significantly different from cells exposed to B[a]P for 8 h (mouse primary hepatocytes) (p < 0.01).
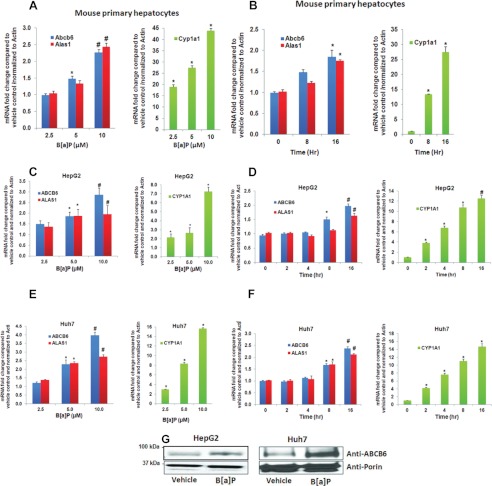
PAH Exposure Induces the Key Regulators of Hepatic Heme Synthesis, ALAS1 and ABCB6/Abcb6
B[a]P- and 3-MC-mediated increase in protoporphyrin IX could be due to either an increase in porphyrin synthesis or a block in porphyrin metabolism or both. To evaluate this further, we first tested whether B[a]P- and 3-MC-induced increase in cellular protoporphyrin IX levels was a result of increased porphyrin biosynthesis. Because hepatic porphyrin biosynthesis is predominantly regulated by the two key regulatory proteins ALAS1 and ABCB6, we measured the expression of these two genes in response to B[a]P and 3-MC as a measure of increased porphyrin biosynthesis. Both time- and dose-dependent effects of B[a]P and 3-MC on porphyrin biosynthesis were evaluated in these studies. In the dose-dependent studies, mouse primary hepatocytes and hepatoma cells were treated with increasing concentrations of B[a]P or 3-MC for 16 h. In the time-dependent studies, cells were exposed to one single dose of 10 μm B[a]P for 0, 2, 4, 8, and 16 h. As shown in Fig. 2, B[a]P induced both ABCB6 and ALAS1 mRNA in a dose- and time-dependent manner in both mouse primary hepatocytes (Fig. 2, A and B) and human hepatoma cells (Fig. 2, C–F). Similarly, 3-MC also induced ABCB6 expression in a dose- and time-dependent manner (supplemental Fig. 1 and data not shown). Significant induction of both of the genes was found at concentrations as low as 5 μm and 16 h of treatment. Further, we found that B[a]P- and 3-MC-mediated increase in ABCB6 mRNA correlated well with B[a]P- and 3-MC-mediated increase in ABCB6 protein expression (Fig. 2G and data not shown). More importantly, the pattern of ABCB6 and ALAS1 induction in response to B[a]P and ABCB6 expression in response to 3-MC correlated well with treatment-mediated increase in hepatic porphyrin levels (Fig. 1 and supplemental Fig. 1). Cytochrome P450 subfamily 1a1 (Cyp1a1) and multidrug resistant protein 4 (Mrp4) genes known to be induced in response to B[a]P and 3-MC, were used as a positive control in these studies (35). Cyp1a1 Mrp4 expression increased with increasing concentration of B[a]P and 3-MC and with increasing time of exposure (Fig. 2 and supplemental Figs. 1 and 3), demonstrating treatment effectiveness. Taken together, results presented in Figs. 1 and 2 and supplemental Fig. 1 demonstrate that B[a]P- and 3-MC-induced hepatic porphyrin levels correlate with an increase in the expression of the key regulators of porphyrin synthesis, ALAS1 and ABCB6.
FIGURE 2.
Benzo[a]pyrene induces ABCB6 and ALAS1 expression in a dose- and time-dependent manner. Exposure to B[a]P induces Abcb6 and Alas1 expression in mouse primary hepatocytes (left panels; A and B) and human hepatomas (left panels; C–F) in a dose-dependent (left panels; A, C, and E) and time-dependent manner (left panels; B, D, and F). Right panels (A–F), B[a]P-mediated induction of CYP1A1. CYP1A1, a gene known to be induced by B[a]P in liver, is used as a positive control in these experiments. G, ABCB6 protein expression in response to B[a]P treatment in human hepatomas. ABCB6 expression was measured in isolated mitochondria using an ABCB6-specific antibody. Values represent mean ± S.D. (error bars) (n = 4). Results shown are representative of three independent experiments with n = 4/experiment. *, significantly different from cells exposed to 2.5 μm B[a]P in dose-dependent studies and significantly different from cells exposed to B[a]P for 4 h (hepatomas) in time-dependent studies (p < 0.01). #, significantly different from cells treated with 5 μm B[a]P in dose-dependent studies and significantly different from 8-h treatment (hepatomas) in time-dependent studies (p < 0.01).
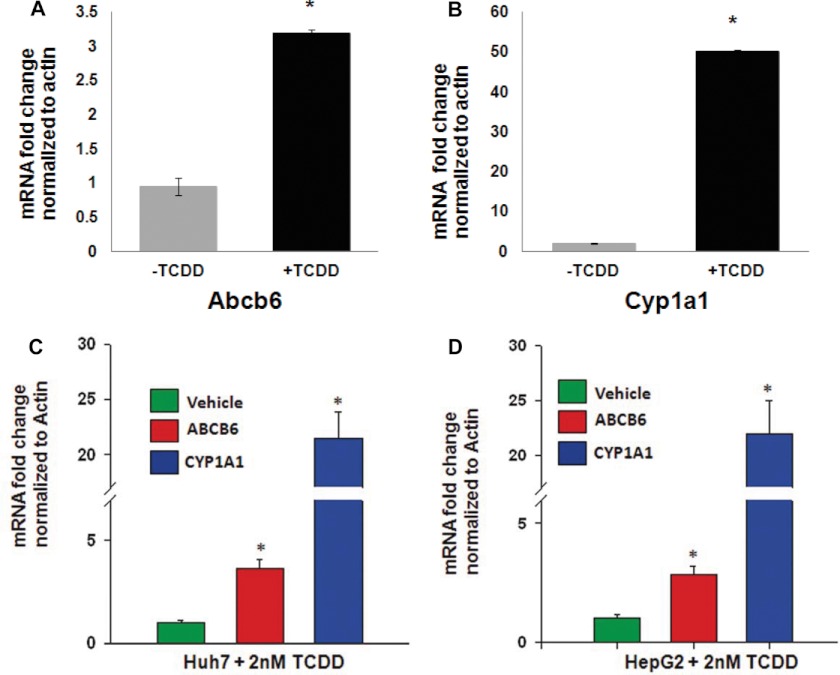
Although TCDD did not increase cellular protoporphyrin IX levels, previous studies have demonstrated that TCDD treatment induces ALAS1 expression probably as a feedback regulation to increased heme demand required for CYP450 activity (36–38). Based on these observations, we hypothesized that TCDD might induce ABCB6 expression similar to what has been reported for ALAS1. To test this hypothesis, we exposed both mice and human hepatoma cells to TCDD and measured ABCB6/Abcb6 expression. Mice were treated with 37 μg/kg/day TCDD for 4 consecutive days in corn oil. In contrast, hepatoma cells were treated with a single dose of 2 nm TCDD for 16 h. We found that TCDD induced ABCB6 expression in both mice (Fig. 3A) and hepatoma cells (Fig. 3, C and D). As before, expression of Cyp1a1, a gene known to be induced in response to TCDD (39, 40), was used as a positive control in these studies. As with B[a]P and 3-MC treatment, Cyp1a1 expression increased with TCDD treatment (Fig. 3, B–D), demonstrating treatment effectiveness. Together these results suggest that TCDD induces ABCB6 expression in a manner similar to its effect on ALAS1 expression.
FIGURE 3.
TCDD induces ABCB6 expression in both mice and humans. Exposure to TCDD induces Abcb6 expression in mouse (A) and human hepatomas (C and D). B, TCDD-mediated induction of Cyp1a1, a gene known to be induced by TCDD in the liver, is used as a positive control in these experiments. Values represent mean ± S.D. (error bars); n = 6 for mice, and n = 4 for cell culture. Results shown are representative of three independent experiments. *, significantly different for vehicle-treated mice in animals and human hepatomas (p < 0.01).
ABCB6 Expression Is Required for PAH-mediated Porphyrin Synthesis
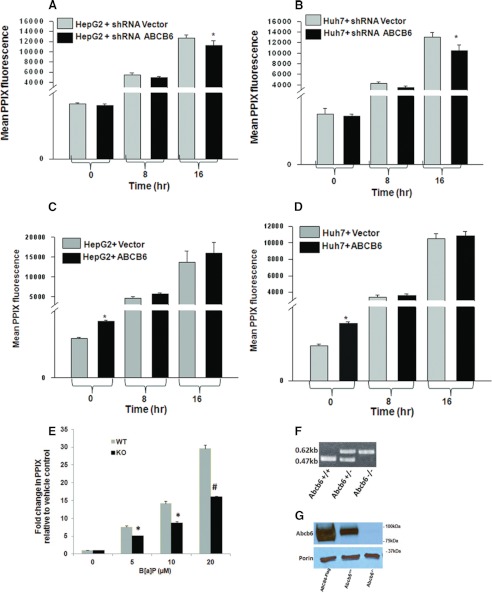
To evaluate the functional importance and the relative contribution of ABCB6 expression to B[a]P induced porphyrin synthesis, we blocked endogenous ABCB6 expression in HepG2 and Huh7 cells and measured porphyrin synthesis following exposure to B[a]P. Reduced ABCB6 expression was achieved in these cells using ABCB6-specific shRNA, which resulted in greater than 80% reduction of endogenous ABCB6 relative to shRNA vector cells (20). Protoporphyrin IX levels were measured in these cells by flow cytometry as described under “Experimental Procedures” and in Ref. 17. We found that reduced ABCB6 expression per se did not decrease protoporphyrin IX levels under physiological conditions, confirming our earlier observations (20) (Fig. 4, A and B). Interestingly, however, in the presence of 5 μm B[a]P, cellular protoporphyrin IX levels were decreased significantly in both of the hepatoma cells (Fig. 4, A and B).
FIGURE 4.
Loss of ABCB6 expression compromises B[a]P-induced hepatic porphyrin levels. Endogenous knockdown of ABCB6 expression results in decreased protoporphyrin IX levels in both HepG2 (A) and Huh7 (B) cells exposed to B[a]P. In contrast, exposure to B[a]P does not affect protoporphyrin IX levels in HepG2 (C) and Huh7 (D) cells overexpressing ABCB6. E, loss of Abcb6 expression results in decreased protoporphyrin IX levels in mouse primary hepatocytes isolated from Abcb6−/− mice exposed to B[a]P. Genotyping (F) and Western blot (G) demonstrate loss of Abcb6 expression in Abcb6−/− mice. Values represent mean ± S.D. (error bars) (n = 3). Results are representative of three independent experiments with n = 3 per experiment. *, significantly different from shRNA vector control cells exposed to B[a]P for 16 h in (A and B) and significantly different from vector control cells (C and D). p < 0.05. E, *, significantly different from B[a]P-treated wild-type mouse primary hepatocytes. p < 0.01. #, significantly different from B[a]P-treated wild-type mouse primary hepatocytes. p < 0.001.
Although a significant decrease in B[a]P-mediated cellular protoporphyrin IX levels was observed in the hepatoma cells in the absence of ABCB6, this decrease was not as dramatic as one would have expected if ABCB6 were to play a significant role in porphyrin biosynthesis. To test this further and to evaluate the full impact of ABCB6 on B[a]P-induced porphyrin biosynthesis, we isolated primary hepatocytes from Abcb6+/+ and Abcb6−/− mice and measured protoporphyrin levels in these hepatocytes in the presence and absence of B[a]P. We found that complete loss of Abcb6 expression significantly compromised the ability of Abcb6−/− primary hepatocytes to respond to B[a]P-mediated increase in cellular protoporphyrin IX levels (Fig. 4E).
We next evaluated the effect of B[a]P treatment on porphyrin biosynthesis in cells engineered to overexpress ABCB6. ABCB6 overexpression was achieved by engineering stable expression of ABCB6 under a constitutively active CMV promoter as described previously (17, 20). In contrast to the porphyrin phenotype seen in response to B[a]P treatment of ABCB6-depleted cells, in ABCB6-overexpressing cells, B[a]P treatment did not increase porphyrin biosynthesis beyond the levels already exhibited by increased ABCB6 overexpression in these cells (Fig. 4, C and D).
Based on the results presented in Fig. 4, A, B, and E, which demonstrate significant decrease in hepatic porphyrin levels in the absence of ABCB6 in response to B[a]P treatment, we hypothesized that B[a]P-induced CYP1A1 activity might be altered in cells that have a loss of ABCB6 expression. However, despite our efforts, we were not able to detect any CYP1A1 activity in these cell culture systems. Thus, evaluation of this aspect requires in vivo studies using Abcb6 gene-deleted mouse.
PAH-mediated Induction of ABCB6/Abcb6 Is AhR-dependent
Recent discoveries have implicated the nuclear receptor AhR in PAH-mediated induction of target genes (41, 42). Thus, in this study, we evaluated the involvement of the AhR pathway in PAH-mediated up-regulation of ABCB6. Toward this end, we examined ABCB6 mRNA expression in response to TCDD and B[a]P treatment both in vivo and in vitro. In the in vivo studies, AhR wild type and AhR knock-out mice were exposed to TCDD as described under “Experimental Procedures.” In the in vitro studies, AhR endogenous knock-down and AhR-expressing hepatoma (HepG2) cells were exposed to B[a]P as described under “Experimental Procedures.” Loss of AhR expression in the AhR knock-out mouse and AhR knock-down cells was confirmed by real-time PCR analysis of the AhR transcript using gene-specific primers (Fig. 5). ABCB6 expression was evaluated by real-time PCR using gene-specific primers. As before, CYP1A1 induction in response to AhR ligands was used as a positive control. We found that in both the AhR knock-out mice and in AhR knock-down hepatoma cells, AhR-responsive gene CYP1A1 was not activated, whereas it was activated in the AhR wild type mouse and hepatoma cells (Fig. 5, B and C). More importantly, we found that TCDD and B[a]P induced a marked increase in the expression of ABCB6 mRNA in AhR-proficient mice (Fig. 5A) and hepatomas (Fig. 5C), but TCDD and B[a]P had no effect on ABCB6 expression in AhR-deficient mice and hepatomas (Fig. 5, A and C). Taken together, these results suggest that PAH-mediated increase in ABCB6/Abcb6 transcript is AhR-dependent.
FIGURE 5.
A functional AhR pathway is required for B[a]P-mediated up-regulation of ABCB6. Mouse (A) and HepG2 (C) cells lacking AhR are unable to up-regulate Abcb6 expression, whereas mouse (A) and HepG2 (C) cells with functional AhR up-regulate Abcb6 expression in response to B[a]P. B, B[a]P-mediated induction of Cyp1a1, a gene known to be induced by B[a]P, is used as a positive control in these experiments. Values represent mean ± S.D. (error bars); n = 4 for mice, and n = 3 for HepG2. Results representative of three independent experiments. *, significantly different from scrambled siRNA transfected cells; p < 0.01 in hepatomas and AhR+/+ vehicle-treated mice. #, significantly different from AhR siRNA-transfected cells treated with B[a]P. p < 0.01.
AhR Activates Transcription of ABCB6 by Binding to the AhRE in the ABCB6/Abcb6 Promoter
Previous studies have demonstrated that in the absence of its ligand, AhR is localized to the cytoplasm. However, upon binding the ligand, AhR translocates from the cytoplasm to the nucleus, where it interacts with specific DNA response elements in target gene promoters and activates gene transcription (43, 44). Thus in these studies, we first confirmed that B[a]P treatment results in the redistribution of AhR from the cytoplasm to the nucleus (supplemental Fig. 2). We next tested whether PAH-induced expression of ABCB6/Abcb6 is mediated by AhR-dependent activation of the ABCB6 promoter. For this purpose, both human and mouse ABCB6 promoters were cloned in frame in a luciferase reporter vector, and the promoter-luciferase-reporter constructs were transfected into HepG2 cells as described (23). Using the heme oxygenase-1 (HO-1) promoter-luciferase-reporter construct, known to be activated by AhR, as a positive control, we evaluated the effect of PAH on the transcriptional activation of the ABCB6/Abcb6 promoter. Because both TCDD and B[a]P are known to activate target genes in a similar manner, via the AhR receptor (43, 44), only B[a]P treatment was used in the promoter activation studies. We found that B[a]P activated both the human and mouse ABCB6 promoter (Fig. 6, A and B) in a manner that corresponded well with ABCB6 transcriptional up-regulation seen in Fig. 2. Together, these results suggest that the B[a]P-mediated increase in ABCB6 expression is mediated by activation of the ABCB6 promoter by AhR.
The apparent similarity in AhR-mediated activation of both the mouse and human ABCB6 promoter suggests similarities in the genomic organization of their AhR-binding response elements. In an effort to identify the existence of such elements in the ABCB6/Abcb6 promoter, we used a computer algorithm (Transfac) to analyze the 5′-flanking sequence of the human and mouse gene encoding ABCB6. Transfac analysis found three putative AhREs located at −102, −115, and −123 bp upstream of the transcription start site in the human and at −168, −185, and −195 bp upstream of the transcription start site in the mouse ABCB6 promoter (Fig. 6C). For clarity, the three AhR binding sites are labeled according to their occurrence in the gene, with the farthest upstream from the transcription start site referred to as ABCB6-ARE1 (−123 bp in the human and −195 bp in the mouse promoter) and the one closest to the start site referred to as ABCB6-ARE3 (−102 bp in the human and −168 bp in the mouse promoter (Fig. 6D)).
The existence of three putative AhREs in the 5′-flanking region of both the human and mouse ABCB6 promoter suggests that AhR could interact with any of these AhREs to induce ABCB6 expression. To test this assertion and to assess the relative contribution of the three AhREs to ABCB6 promoter transactivation by B[a]P, we generated mutations in each of the three AhREs by site-directed mutagenesis (Fig. 6D) and transfected HepG2 cells with these promoter constructs followed by treatment with B[a]P. Because the three AhREs appeared to be conserved in both the mouse and human promoter, and because the activation of these mouse and human promoters appear to be similar (Fig. 6, A and B), only the human promoter was used to explore the relative contribution of the three AhREs to B[a]P-mediated activation. We found that mutations in the two proximal AhREs at −115 and −102 bp (ABCB6-M2 and ABCB6-M3, respectively) did not affect B[a]P-mediated activation of the human ABCB6 promoter (Fig. 6E). In contrast, mutation of the distal AhRE at −123 bp (ABCB6-M1) or mutation of all the three AhREs (ABCB6-M1M2M3) completely abolished B[a]P-mediated activation of the human ABCB6 promoter (Fig. 6E). Taken together, these results suggest that the most distal AhRE in the human ABCB6 promoter (ABCB6-ARE1; at −123 bp) is responsible for B[a]P-mediated induction of ABCB6, whereas the two proximal AhREs (ABCB6-ARE2 and ABCB6-ARE3, at −115 and −102 bp) are dispensable for B[a]P-mediated induction of ABCB6. In all of these studies, the heme oxygenase-1 promoter-luciferase-reporter construct was used as a positive control to confirm treatment effectiveness (Fig. 6).
AhR Binds and Interacts with the Distal AhR Response Element in the ABCB6 Promoter
To confirm and extend our promoter transactivation analysis and to demonstrate that AhR interacts with the distal promoter element of the human ABCB6 promoter, we performed an EMSA with oligonucleotide probe containing the human ABCB6 AhREs. Although both AhREs at −102 and −115 bp failed to activate the ABCB6 promoter in response to B[a]P (Fig. 6E), we decided to include these in our EMSA studies to confirm and extend the results from the promoter transactivation studies (Fig. 6E). Further, because of the close proximity of the AhREs at −115 and −123 bp, a single oligonucleotide probe carrying both of these two sites were used in EMSA. Following EMSA, we found that nuclear extracts of HepG2 cells exposed to B[a]P caused an intense band with the −123 to −115 bp ABCB6-AhRE oligonucleotide probe, retarded on the gel, indicative of a AhRE-AhR protein complex at either −123 or −115 bp (Fig. 7C). In contrast, AhRE at −102 bp did not demonstrate any retarded bands on the gel, suggesting the absence of AhRE-AhR protein complex interaction at the −102 bp site (data not shown). Further, we found that the binding affinity of B[a]P-treated nuclear extract with the oligonucleotide probe (carrying both −123 and −115 bp) could be competed with the use of either excess unlabeled oligonucleotide probe (carrying −123 and −115 bp) or excess unlabeled oligonucleotide probe carrying mutations in the −115 bp AhRE (ABCB6-M2) but not with unlabeled oligonucleotides carrying mutations in ABCB6-M1 (−123 bp) or mutations in both −115 and −123 bp (ABCB6-M1M2) (Fig. 7C). These results suggest that an intact core AhRE at −123 bp in the ABCB6 promoter is required to form this DNA-protein complex. The fact that coincubation with an antibody specific for AhR was capable of reducing the intensity of the −123 bp retarded band on the gel confirms the presence of AhR in this DNA-protein complex (Fig. 7C). Instead of a supershifted band, studies have shown a reduction of AhR/ARNT-response element complex formation in the presence of an AhR antibody (51). Previous studies have demonstrated B[a]P-mediated interaction of AhR with the CYP1A1 AhRE and the ability of AhR-specific antibody to disrupt this interaction. Thus, in our studies, CYP1A1 AhRE interaction with AhR was used as a positive control (Fig. 7A).
Although the EMSA studies demonstrate AhR interaction with the AhRE in the ABCB6 promoter, we observed a significant difference in the migration pattern of ABCB6 promoter-AhR protein complex compared with CYP1A1 promoter-AhR protein complex (Fig. 7, A and C). Further, the intensity of ABCB6 promoter-AhR protein complex on the EMSA was noticeably lighter compared with the interacting complex seen with CYP1A1 (Fig. 7, A and C). We hypothesized that the difference in the migration pattern of the ABCB6-AhR protein complex could be due to DNA binding proteins, activated in response to B[a]P, that interact with the ABCB6 promoter at sites independent of AhR interaction with the AhRE. To test this hypothesis, we synthesized a ABCB6-AhRE oligonucleotide probe where the sequence flanking the AhREs (−123 and −115 bp) was changed to random bases (Fig. 7D) while keeping the core ABCB6-AhRE intact (Fig. 7D). EMSA analysis of the core sequence and the two randomly altered probes demonstrates that changing the AhRE flanking sequence to random bases restores the migration pattern of the ABCB6-AhRE protein complex similar to what was seen with the CYP1A1-AhR protein complex (Fig. 7E). Further, these studies also demonstrate that the AhRE in the ABCB6 promoter is sufficient for AhR interaction with the ABCB6 promoter because changing the flanking sequence did not alter overall AhR binding to the ABCB6-oligonucleotide probe (Fig. 7E). These studies were confirmed further by competition assays using unlabeled and mutant oligonucleotide probes as described above (Fig. 7F). Taken together, our findings suggest that the ABCB6 promoter is directly activated by AhR and that the AhRE at −123 bp (ABCB6-ARE3) is essential and sufficient for the transcriptional activation of the human ABCB6 promoter.
The predicted AhR response element at AhREs in the ABCB6 promoter was further evaluated for its ability to bind AhR in the physiologically relevant cellular environment. For this purpose, we performed chromatin immunoprecipitation experiments in HepG2 cells treated with B[a]P (5 μm). As shown in Fig. 7, G and H, AhR protein was recruited to the AhRE-containing regions of the ABCB6 promoter. Further, the AhR protein interaction with the ABCB6 promoter was significantly increased after B[a]P treatment (Fig. 7, G and H). These results indicate that AhR activates the ABCB6 promoter through direct interaction with the AhREs in the presence of B[a]P in vivo.
DISCUSSION
All PAHs are potent inducers of microsomal monooxygenases, which require heme for their activity (5, 45). Hence, heme demand is higher in animals exposed to PAH (16, 46, 47). When heme demand is high, the preferred response of cells to increased heme demand is to increase heme synthesis. ABCB6 is a mitochondrial transporter that regulates heme synthesis (17). Thus, in this study, we investigated the role of ABCB6 in PAH-mediated increase in heme synthesis and characterized the mechanism that regulates ABCB6 expression under these conditions. This is the first report of the identification of a mammalian xenobiotic response element in the ABCB6/Abcb6 gene.
Previous studies have shown that exposure to TCDD induces the expression of ALAS1, the rate-limiting enzyme in heme biosynthesis, suggesting a feedback regulation of heme synthesis to compensate for the increased heme demand (16). However, this increase in ALAS1 does not result in an increase in heme or its precursor protoporphyrin IX but results in the accumulation of uroporphyrins (36, 37). This is because TCDD inhibits UROD, the enzyme that catalyzes the conversion of uroporphyrinogen to coproporphyrinogen III (36). Our studies confirm these observations showing no increase in protoporphyrin IX levels in mice or tissue culture cells treated with TCDD. However, as with ALAS1 expression, both mouse and human ABCB6 expression were induced in response to TCDD treatment. This response appears to be a coordinated induction mediated by AhR to support the increased expression and activity of induced monooxygenases.
In contrast to TCDD, the effect of B[a]P and 3-MC exposure on hepatic porphyrin levels is not known. We show here that exposure to B[a]P or 3-MC results in increased protoporphyrin levels in both mouse primary hepatocytes and human hepatoma cells, suggesting that the B[a]P and 3-MC effect on UROD activity is not the same as that of TCDD. Our results confirm previous observations in leukocytes, where B[a]P has been show to increase protoporphyrin IX accumulation (48). Again, in response to B[a]P and 3-MC exposure, both ALAS1 and ABCB6 expression were induced, supporting the hypothesis that in response to cellular heme demands, the heme biosynthetic pathway is up-regulated.
In our studies, loss of ABCB6 expression did not alter porphyrin levels in the absence of B[a]P; however, loss of ABCB6 expression significantly compromised B[a]P-mediated induction of hepatic porphyrin levels. These results suggest that under normal physiological conditions, the absence of ABCB6 expression and the ensuing decrease in porphyrin biosynthesis is compensated by a complementary increase in mechanisms that regulate porphyrin synthesis. But when stressed with increased functional demand for heme, the compensatory mechanisms might not be sufficient to complement the loss of ABCB6 expression, thus highlighting the importance of ABCB6 expression in normal and pathophysiological conditions. Recent observations by Ulrich et al. (49) support this hypothesis, demonstrating that in the absence of ABCB6, compensatory mechanisms are activated to compensate for the loss of ABCB6. However, when stressed, loss of ABCB6 compromises cellular heme biosynthesis and function. In this context, it will be interesting to see if loss of ABCB6 expression in vivo (in the Abcb6 gene-deleted animals) compromises CYP450 activity and its effect on drug metabolism and disposition.
A basic pathogenic event in PAH-induced porphyria is the accumulation of porphyrins, in most cases uroporphyrin because of a progressive decrease in UROD activity (36). In many cases, the porphyria remains latent until clinical manifestations (phototoxicity, neural or visceral symptoms, elevation in urinary porphyrins) occur because of pharmacological exposure to chemicals and therapeutic drugs, which precipitates an acute attack because of increased heme synthesis (7, 9, 10, 38, 50). In this regard, increased ABCB6 expression in response to environmental contaminants and therapeutic drugs might contribute to the manifestation of porphyria. In this scenario, increased ABCB6 expression in response to environmental contaminants could enhance heme synthesis in patients compromised in the activity of enzymes of the heme synthetic pathway. This would result in a progressive increase in the accumulation of porphyrin intermediates that leads to clinical manifestation of the latent disease.
Recent studies have demonstrated that the induction of CYP450s by drugs is mediated by several orphan nuclear receptors, members of a superfamily of DNA-binding proteins that act as transcription factors (31). With respect to PAH, the key transcription factor is AhR, which binds the DNA consensus sequence 5′-GCGTG-3′ (also known as AhRE) in the gene promoters, thus inducing transcription. Here we demonstrate that B[a]P-mediated induction of ABCB6 expression is regulated by the AhR pathway. The up-regulation of ABCB6 promoter by AhR and the requirement that a functional AhR be present to ensure up-regulation of ABCB6 expression reveal a novel mechanism whereby a cell can, on demand, induce ABCB6 expression to promote heme biosynthesis to meet the increasing heme demands under these conditions.
In our studies, although three potential AhRE elements were identified by algorithm-based analysis of the ABCB6 promoter, only one of these AhRE elements appeared to be important for AhR-mediated activation of ABCB6. Interestingly, mutation of the AhRE element at −115 bp (ABCB6-M2) showed an increase in promoter activation in response to B[a]P that was significantly higher than the one seen with the unmutated native sequence. We speculate that this observation suggests the potential existence of repressor motifs in close proximity to the AhRE motif at −115 bp. Observations such as these are not uncommon and have given rise to the idea that enhancer and repressor motifs often exist in close proximity to one another and might serve as crucial recruitment sites for transcriptional activation machinery that functions to either promote or suppress gene activation in response to external stimuli. In this context, it is interesting to note that the AhRE sequence 5′-GCGTG-3′ is similar to the HIF-1α response element sequence (consensus 5′-RCGTG-3′), suggesting a potential role of HIF and hypoxia in regulating ABCB6 expression.
It is interesting to note that treatment with B[a]P led to additional DNA-protein complex formation in the ABCB6 promoter. However, this interaction appeared to be independent of AhR interaction with the ABCB6 promoter. At present, it is not clear what these interacting proteins are and what if any is the significance of these interactions to ABCB6 expression and function.
Previous studies have shown that the expression of membrane-bound efflux transporters mediated by AhR in response to environmental contaminants is not uniform between mice and humans (51). For example, breast cancer resistance protein, a transporter important in cellular detoxification and multidrug resistance, is induced in an AhR-dependent manner in humans but not in mice (51). However, in our studies, we found that TCDD and B[a]P induced both mouse and human ABCB6 in an AhR-dependent manner that appears to be comparable, suggesting a common underlying mechanism of PAH-mediated activation of ABCB6 in humans and mice.
The results from our studies presented in this work have both pharmacological and toxicological significance. In addition to their ability to precipitate porphyria, PAHs are carcinogenic in many animal species (26, 29, 52, 53). Following their conversion to dihydrodiol epoxides and epoxide hydrolase by CYP1A1, these compounds interact with DNA and form PAH-DNA adducts (54, 55). These epoxides are highly reactive electrophiles and cause mutations and cytotoxicity in both prokaryotic and eukaryotic cells. In this context, it is interesting to note that ABCB6 expression promotes cell growth and proliferation in hepatoma cells, and ABCB6 expression is induced during the development of hepatocellular carcinoma (20, 56, 57). Thus, ABCB6 induction by PAHs could be a potential contributing factor in PAH carcinogenicity. In addition, it is also possible that PAH-mediated activation of ABCB6 could enhance CYP1A1 activity and accelerate the metabolism of PAHs to epoxide hydrolase, thus promoting carcinogenesis.
In conclusion, this is the first report to illustrate that an important regulator of heme synthesis, ABCB6, is transcriptionally regulated by AhR. The resultant enhancement of ABCB6 function represents an essential requirement coordinated by AhR to support the increased heme demand that occurs when animals are exposed to xenobiotics and therapeutic drugs. The observation that this AhR-induced expression of ABCB6 is common to both mice and humans highlights the importance and significance of this process.
Acknowledgments
We thank Dr. Hongbing Wang (University of Maryland) for AhR siRNA, Dr. Curtis Klaassen for the AhR wild type and gene-deleted mouse models, and Dr. Kathy Roby for the AhR antibody. We acknowledge Dr. Bryan Copple, Dr. Jim Luyendyk, and Dr. Wen-Xing Ding for technical help in isolating and culturing mouse primary hepatocytes and Dr. Andrew Lickteig for technical assistance with the mice TCDD studies.
This work was supported, in whole or in part, by National Institutes of Health Grants P20RR021940 and T32ES007079.

This article contains supplemental Figs. 1–4.
H. Chavan and P. Krishnamurthy, manuscript in preparation.
- CYP450
- cytochrome P450
- AhR
- aryl hydrocarbon receptor
- AhRE
- aryl hydrocarbon-responsive element
- ALAS
- 5-aminolevulinic acid synthase
- B[a]P
- benzo[a]pyrene
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- 3-MC
- 3-methylcholanthrene
- PAH
- polyaromatic hydrocarbon.
REFERENCES
- 1. Ponka P. (1999) Cell biology of heme. Am. J. Med. Sci. 318, 241–256 [DOI] [PubMed] [Google Scholar]
- 2. Padmanaban G., Venkateswar V., Rangarajan P. N. (1989) Heme as a multifunctional regulator. Trends Biochem. Sci. 14, 492–496 [DOI] [PubMed] [Google Scholar]
- 3. Sassa S., Nagai T. (1996) The role of heme in gene expression. Int. J. Hematol. 63, 167–178 [DOI] [PubMed] [Google Scholar]
- 4. Aft R. L., Mueller G. C. (1984) Hemin-mediated oxidative degradation of proteins. J. Biol. Chem. 259, 301–305 [PubMed] [Google Scholar]
- 5. Maines M. D., Kappas A. (1975) The degradative effects of porphyrins and heme compounds on components of the microsomal mixed function oxidase system. J. Biol. Chem. 250, 2363–2369 [PubMed] [Google Scholar]
- 6. Bian K., Gao Z., Weisbrodt N., Murad F. (2003) The nature of heme/iron-induced protein tyrosine nitration. Proc. Natl. Acad. Sci. U.S.A. 100, 5712–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sassa S. (2000) Hematologic aspects of the porphyrias. Int. J. Hematol 71, 1–17 [PubMed] [Google Scholar]
- 8. Granick S., Sinclair P., Sassa S., Grieninger G. (1975) Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J. Biol. Chem. 250, 9215–9225 [PubMed] [Google Scholar]
- 9. Lindberg R. L., Porcher C., Grandchamp B., Ledermann B., Bürki K., Brandner S., Aguzzi A., Meyer U. A. (1996) Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat. Genet. 12, 195–199 [DOI] [PubMed] [Google Scholar]
- 10. Sassa S. (2002) The porphyrias. Photodermatol. Photoimmunol. Photomed. 18, 56–67 [DOI] [PubMed] [Google Scholar]
- 11. May B. K., Dogra S. C., Sadlon T. J., Bhasker C. R., Cox T. C., Bottomley S. S. (1995) Molecular regulation of heme biosynthesis in higher vertebrates. Prog. Nucleic Acid Res. Mol. Biol. 51, 1–51 [DOI] [PubMed] [Google Scholar]
- 12. Riddle R. D., Yamamoto M., Engel J. D. (1989) Expression of δ-aminolevulinate synthase in avian cells. Separate genes encode erythroid-specific and nonspecific isozymes. Proc. Natl. Acad. Sci. U.S.A. 86, 792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schoenhaut D. S., Curtis P. J. (1986) Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene 48, 55–63 [DOI] [PubMed] [Google Scholar]
- 14. Munakata H., Yamagami T., Nagai T., Yamamoto M., Hayashi N. (1993) Purification and structure of rat erythroid-specific δ-aminolevulinate synthase. J. Biochem. 114, 103–111 [DOI] [PubMed] [Google Scholar]
- 15. Labbe-Bois R., Simon M., Rytka J., Litwinska J., Bilinski T. (1980) Effect of 5-aminolevulinic acid synthesis deficiency on expression of other enzymes of heme pathway in yeast. Biochem. Biophys. Res. Commun. 95, 1357–1363 [DOI] [PubMed] [Google Scholar]
- 16. Jover R., Hoffmann K., Meyer U. A. (1996) Induction of 5-aminolevulinate synthase by drugs is independent of increased apocytochrome P450 synthesis. Biochem. Biophys. Res. Commun. 226, 152–157 [DOI] [PubMed] [Google Scholar]
- 17. Krishnamurthy P. C., Du G., Fukuda Y., Sun D., Sampath J., Mercer K. E., Wang J., Sosa-Pineda B., Murti K. G., Schuetz J. D. (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443, 586–589 [DOI] [PubMed] [Google Scholar]
- 18. Lynch J., Fukuda Y., Krishnamurthy P., Du G., Schuetz J. D. (2009) Cell survival under stress is enhanced by a mitochondrial ATP-binding cassette transporter that regulates hemoproteins. Cancer Res. 69, 5560–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nilsson R., Schultz I. J., Pierce E. L., Soltis K. A., Naranuntarat A., Ward D. M., Baughman J. M., Paradkar P. N., Kingsley P. D., Culotta V. C., Kaplan J., Palis J., Paw B. H., Mootha V. K. (2009) Discovery of genes essential for heme biosynthesis through large scale gene expression analysis. Cell Metab. 10, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polireddy K., Chavan H., Abdulkarim B. A., Krishnamurthy P. (2011) Functional significance of the ATP-binding cassette transporter B6 in hepatocellular carcinoma. Mol. Oncol. 5, 410–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chavan H., Oruganti M., Krishnamurthy P. (2011) The ATP-binding cassette transporter ABCB6 is induced by arsenic and protects against arsenic cytotoxicity. Toxicol. Sci. 120, 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Copple B. L. (2010) Hypoxia stimulates hepatocyte epithelial to mesenchymal transition by hypoxia-inducible factor and transforming growth factor-β-dependent mechanisms. Liver Int. 30, 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishnamurthy P., Ross D. D., Nakanishi T., Bailey-Dell K., Zhou S., Mercer K. E., Sarkadi B., Sorrentino B. P., Schuetz J. D. (2004) The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 279, 24218–24225 [DOI] [PubMed] [Google Scholar]
- 24. Tompkins L. M., Li H., Li L., Lynch C., Xie Y., Nakanishi T., Ross D. D., Wang H. (2010) A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem. Pharmacol. 80, 1754–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shou M., Harvey R. G., Penning T. M. (1993) Reactivity of benzo[a]pyrene-7,8-dione with DNA. Evidence for the formation of deoxyguanosine adducts. Carcinogenesis 14, 475–482 [DOI] [PubMed] [Google Scholar]
- 26. Oya E., Ovrevik J., Arlt V. M., Nagy E., Phillips D. H., Holme J. A. (2011) DNA damage and DNA damage response in human bronchial epithelial BEAS-2B cells following exposure to 2-nitrobenzanthrone and 3-nitrobenzanthrone. Role in apoptosis. Mutagenesis 26, 697–708 [DOI] [PubMed] [Google Scholar]
- 27. Detmar J., Rennie M. Y., Whiteley K. J., Qu D., Taniuchi Y., Shang X., Casper R. F., Adamson S. L., Sled J. G., Jurisicova A. (2008) Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am. J. Physiol. Endocrinol. Metab. 295, E519–E530 [DOI] [PubMed] [Google Scholar]
- 28. Mandal P. K. (2005) Dioxin. A review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B 175, 221–230 [DOI] [PubMed] [Google Scholar]
- 29. Boffetta P., Mundt K. A., Adami H. O., Cole P., Mandel J. S. (2011) TCDD and cancer. A critical review of epidemiologic studies. Crit. Rev. Toxicol. 41, 622–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malkinson A. M. (1983) Review. Putative mutagens and carcinogens in foods. III. Butylated hydroxytoluene (BHT). Environ. Mutagen. 5, 353–362 [DOI] [PubMed] [Google Scholar]
- 31. Honkakoski P., Negishi M. (2000) Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem. J. 347, 321–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei P., Zhang J., Egan-Hafley M., Liang S., Moore D. D. (2000) The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407, 920–923 [DOI] [PubMed] [Google Scholar]
- 33. Ueda A., Hamadeh H. K., Webb H. K., Yamamoto Y., Sueyoshi T., Afshari C. A., Lehmann J. M., Negishi M. (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 61, 1–6 [DOI] [PubMed] [Google Scholar]
- 34. Maglich J. M., Stoltz C. M., Goodwin B., Hawkins-Brown D., Moore J. T., Kliewer S. A. (2002) Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 62, 638–646 [DOI] [PubMed] [Google Scholar]
- 35. Whitlock J. P., Jr. (1999) Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 39, 103–125 [DOI] [PubMed] [Google Scholar]
- 36. Cantoni L., Rizzardini M., Graziani A., Carugo C., Garattini S. (1987) Effects of chlorinated organics on intermediates in the heme pathway and on uroporphyrinogen decarboxylase. Ann. N.Y. Acad. Sci. 514, 128–140 [DOI] [PubMed] [Google Scholar]
- 37. Lambrecht R. W., Sinclair P. R., Bement W. J., Sinclair J. F. (1988) Uroporphyrin accumulation in cultured chick embryo hepatocytes. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,4,3′,4′-tetrachlorobiphenyl. Toxicol. Appl. Pharmacol. 96, 507–516 [DOI] [PubMed] [Google Scholar]
- 38. Davies R., Clothier B., Robinson S. W., Edwards R. E., Greaves P., Luo J., Gant T. W., Chernova T., Smith A. G. (2008) Essential role of the AH receptor in the dysfunction of heme metabolism induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem. Res. Toxicol. 21, 330–340 [DOI] [PubMed] [Google Scholar]
- 39. Rifkind A. B. (2006) CYP1A in TCDD toxicity and in physiology, with particular reference to CYP-dependent arachidonic acid metabolism and other endogenous substrates. Drug Metab. Rev. 38, 291–335 [DOI] [PubMed] [Google Scholar]
- 40. Nebert D. W., Jones J. E. (1989) Regulation of the mammalian cytochrome P1–450 (CYP1A1) gene. Int. J. Biochem. 21, 243–252 [DOI] [PubMed] [Google Scholar]
- 41. Steinmetz A. C., Renaud J. P., Moras D. (2001) Binding of ligands and activation of transcription by nuclear receptors. Annu. Rev. Biophys. Biomol. Struct. 30, 329–359 [DOI] [PubMed] [Google Scholar]
- 42. Stejskalova L., Dvorak Z., Pavek P. (2011) Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr. Drug Metab. 12, 198–212 [DOI] [PubMed] [Google Scholar]
- 43. Beischlag T. V., Luis Morales J., Hollingshead B. D., Perdew G. H. (2008) The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 18, 207–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Safe S., Krishnan V. (1995) Cellular and molecular biology of aryl hydrocarbon (Ah) receptor-mediated gene expression. Arch. Toxicol. Suppl. 17, 99–115 [DOI] [PubMed] [Google Scholar]
- 45. Zhu Y., Silverman R. B. (2008) Revisiting heme mechanisms. A perspective on the mechanisms of nitric-oxide synthase (NOS), heme oxygenase (HO), and cytochrome P450s (CYP450s). Biochemistry 47, 2231–2243 [DOI] [PubMed] [Google Scholar]
- 46. Fraser D. J., Podvinec M., Kaufmann M. R., Meyer U. A. (2002) Drugs mediate the transcriptional activation of the 5-aminolevulinic acid synthase (ALAS1) gene via the chicken xenobiotic-sensing nuclear receptor (CXR). J. Biol. Chem. 277, 34717–34726 [DOI] [PubMed] [Google Scholar]
- 47. Fraser D. J., Zumsteg A., Meyer U. A. (2003) Nuclear receptors constitutive androstane receptor and pregnane X receptor activate a drug-responsive enhancer of the murine 5-aminolevulinic acid synthase gene. J. Biol. Chem. 278, 39392–39401 [DOI] [PubMed] [Google Scholar]
- 48. Uribe-Hernández R., Pérez-Zapata A. J., Vega-Barrita M. L., Ramón-Gallegos E., Amezcua-Allieri M. A. (2008) Cell metabolic changes of porphyrins and superoxide anions by anthracene and benzo[a]pyrene. Environ. Toxicol. Pharmacol. 26, 237–240 [DOI] [PubMed] [Google Scholar]
- 49. Ulrich D. L., Lynch J., Wang Y., Fukuda Y., Nachagari D., Du G., Sun D., Fan Y., Tsurkan L., Potter P. M., Rehg J. E., Schuetz J. (2012) ATP-dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity. J. Biol. Chem. 287, 12679–12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sassa S., Kappas A. (2000) Molecular aspects of the inherited porphyrias. J. Intern. Med. 247, 169–178 [DOI] [PubMed] [Google Scholar]
- 51. Tan K. P., Wang B., Yang M., Boutros P. C., Macaulay J., Xu H., Chuang A. I., Kosuge K., Yamamoto M., Takahashi S., Wu A. M., Ross D. D., Harper P. A., Ito S. (2010) Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2). Mol. Pharmacol. 78, 175–185 [DOI] [PubMed] [Google Scholar]
- 52. Giles A. S., Seidel A., Phillips D. H. (1996) Covalent DNA adducts formed in mouse epidermis by benzo[g]chrysene. Carcinogenesis 17, 1331–1336 [DOI] [PubMed] [Google Scholar]
- 53. Knerr S., Schrenk D. (2006) Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 50, 897–907 [DOI] [PubMed] [Google Scholar]
- 54. Salas V. M., Burchiel S. W. (1998) Apoptosis in Daudi human B cells in response to benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol. Toxicol. Appl. Pharmacol. 151, 367–376 [DOI] [PubMed] [Google Scholar]
- 55. Lin T., Yang M. S. (2008) Benzo[a]pyrene-induced necrosis in the HepG(2) cells via PARP-1 activation and NAD(+) depletion. Toxicology 245, 147–153 [DOI] [PubMed] [Google Scholar]
- 56. Hlavata I., Mohelnikova-Duchonova B., Vaclavikova R., Liska V., Pitule P., Novak P., Bruha J., Vycital O., Holubec L., Treska V., Vodicka P., Soucek P. (2012) The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 27, 187–196 [DOI] [PubMed] [Google Scholar]
- 57. Borel F., Han R., Visser A., Petry H., van Deventer S. J., Jansen P. L., Konstantinova P. (2012) Adenosine triphosphate-binding cassette transporter gene up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology 55, 821–832 [DOI] [PubMed] [Google Scholar]