FIGURE 6.
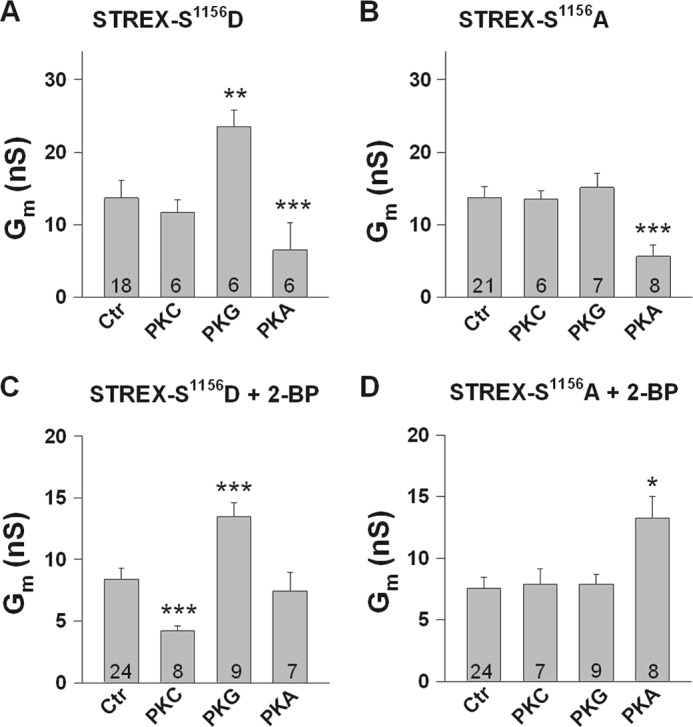
Phosphorylation of serine 1156 determines the sensitivity of STREX channels to protein kinases. A–D, bars represent conductances at +20 mV from inside-out membrane patches obtained from HEK293 cells expressing either the phosphomimetic S1156D (A and C) or the phosphoresistant S1156A STREX mutant channels (B and D). Cells in C and D were treated with the palmitoyl transferase inhibitor 2-BP (100 μm). Phosphorylation of Ser1156 is a precondition for PKG-dependent STREX activation (A and B) and for PKCc-dependent STREX inhibition which, however, occurs only when STREX is detached from the plasma membrane (+2-BP in C and D). The PKA-induced inhibition of STREX is independent of Ser1156 (A and B) but relies on the membrane-attached STREX insert (C). In the S1156A mutant that mimics the dephosphorylated channel at this position, PKAc enhances Gm when STREX is detached from the membrane (+2-BP in D). PKCc was applied at 20 nm and PKG and PKAc at 300 nm, respectively. Means ± S.E. are shown. Numbers within bars represent the number of membrane patches. The intracellular (bath) Ca2+ concentration was 1 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
