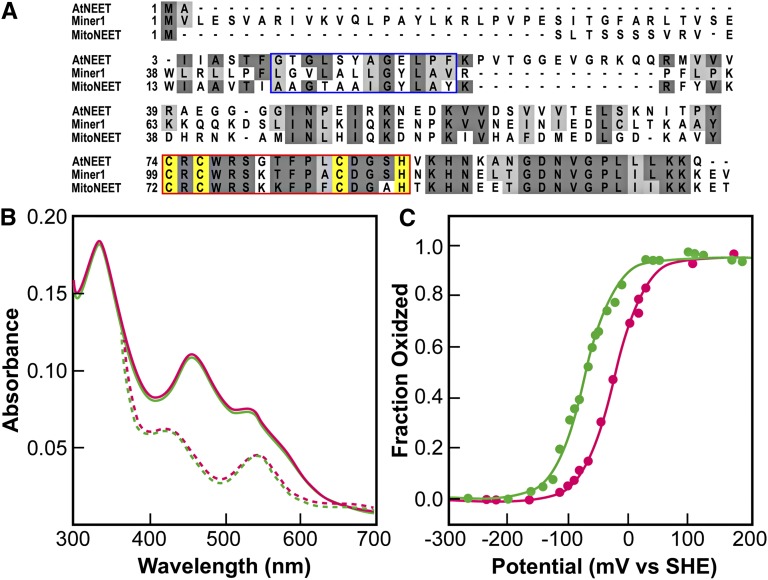
Figure 1.
The At5g51720 Protein Exhibits Similar Properties to Human NEET Proteins.
(A) Alignment of the Arabidopsis At5g51720 protein with the human mitoNEET and Miner1 proteins. The blue box highlights the transmembrane region identified in the human proteins. The red box highlights the conserved 2Fe-2S cluster binding domain. The cluster binding side chains for the proteins are highlighted in yellow.
(B) UV-Vis optical spectra of At5g51720 (green) and human mitoNEET (magenta). The peaks at 458 and 530 nm (solid traces) are characteristic of ligand-to-charge transfer bands of the 2Fe-2S centers in human mitoNEET (Paddock et al., 2007; Wiley et al., 2007b). Upon addition of the reducing dithionite, the spectrum (reversibly) changed with peaks near 440 and 550 nm (dotted traces), nearly identical to that of the reduced human mitoNEET.
(C) Redox potential (EM) of the 2Fe-2S clusters of At5g51720 (green) and human mitoNEET (magenta) at pH 7.0. The EM of At5g51720 was measured using potentiometric redox titrations. The value for EM at pH 7.0 (EM, 7) was ?50 mV lower than that of human mitoNEET. At5g51720’s EM was found to be pH dependent (see Supplemental Figure 1 online), with a slope of −50 mV/pH.