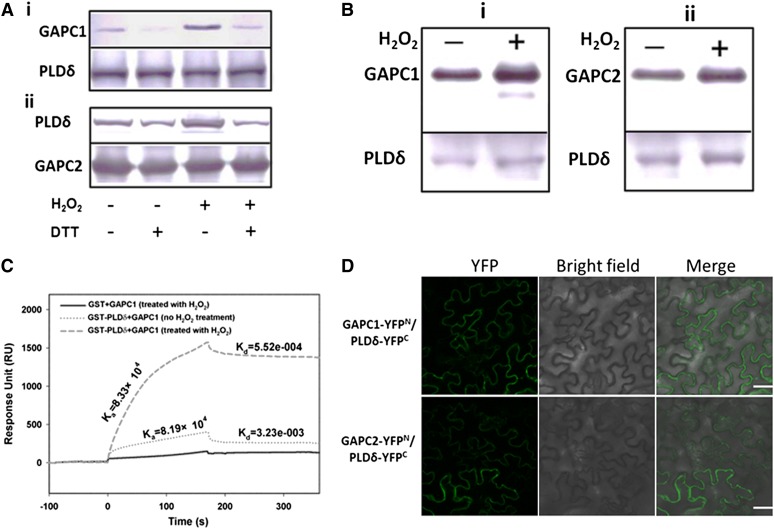
Figure 2.
Interaction of GAPC with PLDδ.
(A) Immunoblotting of proteins after coprecipitation using E. coli–expressed GST-PLDδ and His-GAPC1/2, as affected by H2O2 (100 μM) and DTT (100 μM). i, Coprecipitation of His-GAPC1 with GST-PLDδ. GAPC1, immunoblotting of GAPC1 using anti-His antibody for the precipitates; PLDδ, the starting GST-PLDδ used for precipitation. ii, Coprecipitation of GST-PLDδ with His-GAPC2. PLDδ, immunoblotting of PLDδ using anti-GST antibody for the precipitates. GAPC2, the starting His-GAPC2 used for precipitation. DTT was added before the addition of H2O2 when both were applied.
(B) Immunoblotting of coprecipitated GAPC and PLDδ that were coexpressed in yeast grown in the presence or absence of added H2O2 (20 μM). i and ii, Reciprocal pulldown of PLDδ and GAPC1 and GAPC2, respectively. PLDδ was fused with a FLAG tag and GAPC1or GAPC2 with a cMyc tag. GAPC1 or GAPC2 band indicates immunoblotting with cMyc antibody against the sample precipitated with FLAG antibody–conjugated agarose beads. PLDδ band indicates immunoblotting with FLAG antibody against the sample precipitated with cMyc antibody for GAPC1 or GAPC2.
(C) Quantitative SPR analysis of PLDδ binding to GAPC1. GAPC1 (no H2O2 treatment or pretreated with 100 µM H2O2) was first immobilized on the NTA chip followed by injection of GST or GST-PLDδ.
(D) Representative confocal images of BiFC. Green color represents YFP fluorescence, indicating interaction of GAPC with PLDδ. PLDδ-YFPC was cotransformed with GAPC1-YFPN or GAPC2-YFPN into tobacco leaves by infiltration. Bars = 50 µm.