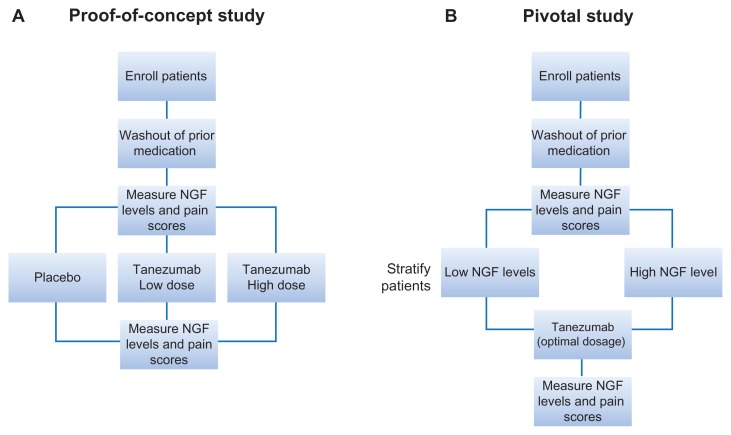
Figure 2.
Proposed study schema to assess NGF-dependence of tanezumab efficacy. (A) Proof-of-concept study in which NGF is measured in EPS and urine before and after treatment with placebo or tanezumab at two different doses. (B) Pivotal study in which NGF levels are measured at pre- and posttreatment time points to establish NGF levels as a predictive biomarker for anti-NGF therapy.
Abbreviations: EPS, expressed prostatic secretion; NGF, nerve growth factor.